Abstract
Therapeutic hypothermia (TH) has become a standard of care following hypoxic ischemic encephalopathy (HIE). After TH, body temperature is brought back to 37 °C over 14 h. Lactate/N-acetylasperatate (Lac/NAA) peak area ratio on proton magnetic resonance spectroscopy (1H MRS) is the best available outcome biomarker following HIE. We hypothesized that broadband near infrared spectroscopy (NIRS) measured changes in the oxidation state of cytochrome-c-oxidase concentration (Δ[oxCCO]) and cerebral hemodynamics during rewarming would relate to Lac/NAA. Broadband NIRS and systemic data were collected during rewarming from 14 infants following HIE over a mean period of 12.5 h. 1H MRS was performed on day 5–9. Heart rate increased by 20/min during rewarming while blood pressure and peripheral oxygen saturation (SpO2) remained stable. The relationship between mitochondrial metabolism and oxygenation (measured as Δ[oxCCO] and Δ[HbD], respectively) was calculated by linear regression analysis. This was reviewed in three groups: Lac/NAA values <0.5, 0.5–1, >1. Mean regression coefficient (r 2) values in these groups were 0.41 (±0.27), 0.22 (±0.21) and 0.01, respectively. The relationship between mitochondrial metabolism and oxygenation became impaired with rising Lac/NAA. Cardiovascular parameters remained stable during rewarming.
The original version of this chapter was revised. An erratum to this chapter can be found at DOI 10.1007/978-3-319-38810-6_59
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Hypoxic-ischaemic brain injury
- Cerebral oxygenation
- Cerebral metabolism
- Cytochrome-c-oxidase
- Newborn infant
1 Introduction
Perinatal hypoxic-ischaemic (HI ) brain injury causes significant morbidity and mortality. Therapeutic hypothermia (TH) is beneficial following hypoxic ischemic encephalopathy (HIE) and has become the standard of care in recent years [1, 2]. During TH, body temperature is maintained at 33.5 °C followed by a slow rewarming that brings body temperature back to 37 °C. Rewarming early from TH induces cortical neuron apoptosis in a piglet model following HIE [3]. Rebound seizures have been noted during the rewarming period both in animal models [4] and neonatal intensive care [5] and further ‘cooling’ with slower rewarming has been suggested. Changes in cerebral metabolism and hemodynamics have been investigated in both preclinical models and clinical studies during and after HI , but the dynamic effects of rewarming on newborn cerebral metabolism and hemodynamics have not yet been fully investigated.
NIRS is a non-invasive tool that has been widely used for continuous bedside monitoring of cerebral oxygenation and hemodynamic changes. We have recently developed a new broadband NIRS system to monitor Δ[oxCCO] as well as the concentration changes of oxy- and deoxy hemoglobin (Δ[HbO2] and Δ[HHb], respectively) in neonatal brain [6]. Cytochrome-c-oxidase (CCO) plays a crucial role in mitochondrial oxidative metabolism and ATP synthesis and is responsible for more than 95 % of oxygen metabolism in the body [7]. Changes in total hemoglobin (HbT = HbO2 + HHb) and hemoglobin difference (HbD = HbO2 − HHb) were calculated. Changes in HbD and HbT reflect changes in cerebral oxygenation and changes in cerebral blood volume, respectively.
Following HIE, Lac/NAA peak area ratio obtained from 1H MRS is the best available MR biomarker for prediction of neurodevelopmental outcome [8].
The aim of this study was to assess the cerebral metabolic and hemodynamic changes during the rewarming period in a cohort of term infants following perinatal hypoxic-ischemic brain injury. We hypothesized that the dynamic changes in cerebral oxygenation and metabolism during the rewarming period would relate to the severity of the injury as assessed by Lac/NAA.
2 Methods
Ethical approval for the study at University College London Hospitals NHS Foundation Trust (UCLH) was obtained from the NREC (reference: 13/LO/0225). Hypothermia and subsequent rewarming were instituted with a servo-controlled cooling machine Tecotherm Neo, Inspiration Healthcare, UK. Using a cooling mattress and a rectal temperature probe, it maintains a constant core temperature during hypothermia and increases the temperature as programmed during the rewarming period. Normally, the core temperature is increased by 0.5 °C over every 2-h period so that the temperature is increased from 33.5 to 37 °C over 14 h. Rewarming data were collected from a cohort of 14 infants. NIRS monitoring was commenced as early as possible in the rewarming phase and was continued for the maximum possible time. One NIRS channel was placed on either side of the forehead and data were collected at 1 Hz. The optode source-detector distance of 2.5 cm was chosen to ensure a good depth penetration [9]. The differential path length (DPF) was chosen as 4.99 [10]. Changes in chromophore concentrations were calculated from the measured changes in broadband NIR light attenuation using the modified Beer-Lambert law as applied with the UCLn algorithm [11] across 136 wavelengths (770–906 nm). Systemic data were collected using ixTrend software (ixellence GmbH, Germany) and were synchronised with the NIRS data. Brain magnetic resonance imaging (MRI) and MRS were performed between day 5–9 using a 3T Philips MRI scanner (Philips Healthcare, UK).
3 Data Analysis
Initial data analysis was carried out in MATLAB (Mathworks, USA). Systemic data were down-sampled and interpolated to the NIRS data timeframe (1 Hz). Artefacts from movement or changes in external lighting were removed and baseline shifts were corrected using the method suggested by Scholkmann et al. [12]. Linear regression analysis was performed to assess the relationship between Δ[oxCCO] and Δ[HbD] and an averaged regression coefficient (r 2) was created to compare this relationship between groups. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, USA).
4 Results
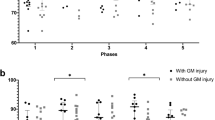
NIRS and systemic data were collected over a mean period of 12.5 h (5–14 h). Active cooling was started at a mean of 3 h of age. All infants were ventilated during rewarming. Clinical characteristics of the cohort are presented in Table 33.1. During rewarming the heart rate gradually increased from a mean of 108/min (range 75–130/min) to 128/min (121–135/min). The peripheral oxygen saturation (SpO2) fell briefly at the start of rewarming , but was mostly over 95 %. Mean blood pressure (BP) was stable throughout the rewarming (45–55 mmHg) (Fig. 33.1). Transcutaneous CO2 dropped by mean 1.3 kPa. Changes in oxCCO concentration from both left and right side are shown in Fig. 33.2. No significant difference was noted between the two sides. The averaged regression coefficients (r 2) between Δ[oxCCO] and Δ[HbD] were plotted against Lac/NAA values obtained from 1H MRS. Although in clinical practice a Lac/NAA peak area ratio >0.3 has been associated with poor neurodevelopmental outcome following HIE [8], we divided the current cohort into three groups (Lac/NAA <0.5, 0.5–1 and >1) to more explicitly demonstrate the relationship between the average regression coefficient between Δ[oxCCO] and Δ[HbD] with Lac/NAA . Lac/NAA peak area ratio ranged from 0.08 to 1.32 in this cohort, and four infants had a value of ≥0.3. Eleven infants had Lac/NAA <0.5, 2 had Lac/NAA 0.5–1 and 1 had Lac/NAA > 1. Mean r 2 values between Δ[oxCCO] and Δ[HbD] in these groups were 0.41 (±0.27), 0.22 (±0.21) and 0.01, respectively (Fig. 33.3).
5 Discussion
The relationship between cerebral metabolism and oxygenation measured during rewarming following TH in a group of infants with HIE became more disturbed with an increasing degree of brain injury. Rewarming is a complex process; it has the potential to significantly influence cardiovascular function and stimulate the activity of excitatory amino acids suppressed during hypothermia [13].
Gebaur et al. reported that during hypothermia left ventricular output remains low due to decreased heart rate and a decreased stroke volume. During rewarming both stroke volume and cardiac output increases as the core temperature increases [14]. In our study, heart rate increased steadily throughout rewarming but mean BP remained stable, no hypotensive episodes were noted. Our findings were similar to those of Gebauer et al. but were different from those reported by Thoresen and Whitlaw [15]. They noted changes in cardiovascular indices in 9 infants with HIE during mild hypothermia and rewarming. Mean arterial BP fell during rewarming while the heart rate increased. This difference is probably related to the process of rewarming. In the study described by Thoresen et al. rewarming was performed over a minimum of 5 h by removing the cooling cap and adjusting the overhead heater to control the rise of temperature no more than per hour. We used a servo controlled cooling machine, which increased the core temperature in a more stable way, and the rewarming was done over a much longer period. A rapid and unstable increase in core temperature most likely induces a reduced peripheral vascular tone with increased cardiac work. Rewarming at a rate of 0.5–1 °C/h did not influence the beneficial effects of therapeutic hypothermia in a rat model, but a higher rewarming rate of 2 °C/h abolished those beneficial effects on both cardiac and cerebral function (reduced severity of myocardial and cerebral functions abnormalities and attenuated release of IL-6 and TNF-α) [16].
Availability of oxygen has a significant influence on the oxidation of CCO. The relationship between mitochondrial metabolism and oxygenation became more impaired with increasing severity of injury, measured as rising Lac/NAA on 1H MRS. This probably indicates that following severe HI E and cell death, cerebral metabolism failed to improve in spite of oxygen availability. We did not notice any significant pattern of changes in Δ[oxCCO] with a change in temperature, nor did we notice any specific cut-off temperature point which indicated any change in the trend of cerebral metabolism during rewarming. In near-term fetal sheep, carotid artery blood flow (CaBF) and mean arterial blood pressure (MABP) changed only transiently during rewarming. No significant difference was noted from 6 h onwards [3]. In a recent study, asphyxiated newborn infants had stable regional cerebral oxygenation during rewarming [17].
The limitation of the present study is the small number of infants enrolled and, in particular, the number of infants with severe brain injury.
We noted that the mitochondrial metabolism-oxygenation coupling during rewarming was influenced by the severity of hypoxic ischemic brain injury . Servo-controlled slow rewarming process had no significant influence on the stability of cerebrovascular hemodynamics and metabolism.
References
NICE (2010) Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury. (http://www.nice.org.uk/guidance/ipg347/chapter/1-guidance)
Committee on Fetus and Newborn. American Academy of Pediatrics (2014) Hypothermia and neonatal encephalopathy. Pediatrics 133(6):1146–1150
Wang B, Armstrong JS, Lee JH et al (2015) Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab 35(5):781–793
Gerrits LC, Battin MR, Bennet L et al (2005) Epileptiform activity during rewarming from moderate cerebral hypothermia in the near-term fetal sheep. Pediatr Res 57(3):342–346
Kendall GS, Mathieson S, Meek J et al (2012) Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics 130(2):e451–e455
Bale G, Mitra S, Meek J et al (2014) A new broadband near-infrared spectroscopy system for in-vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed Optics Express 5(10):3450–3466
Richter OM, Ludwig B (2003) Cytochrome c oxidase—structure, function, and physiology of a redox-driven molecular machine. Rev Physiol Biochem Pharmacol 147:47–74
Thayyil S, Chandrasekaran M, Taylor A et al (2010) Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125(2):e382–e395
Grant PE, Roche-Labarbe N, Surova A et al (2009) Increased cerebral blood volume and oxygen consumption in neonatal brain injury. J Cereb Blood Flow Metab 29(10):1704–1713
Duncan A, Meek JH, Clemence M et al (1996) Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res 39:889–894
Matcher S, Elwell C, Cooper C (1995) Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal Biochem 227(1):54–68
Scholkmann F, Spichtig S, Muehlemann T et al (2010) How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol Meas 31(5):649–662
Nakashima K, Todd MM (1996) Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 27(5):913–918
Gebauer CM, Knuepfer M, Robel-Tillig E, Pulzer F, Vogtmann C (2006) Hemodynamics among neonates with hypoxic-ischemic encephalopathy during whole-body hypothermia and passive rewarming. Pediatrics 117(3):843–850
Thoresen M, Whitelaw A (2000) Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 106(1 Pt 1):92–99
Lu X, Ma L, Sun S et al (2014) The effects of the rate of postresuscitation rewarming following hypothermia on outcomes of cardiopulmonary resuscitation in a rat model. Crit Care Med 42(2):e106–e113
Peng S, Boudes E, Tan X et al (2015) Does near-infrared spectroscopy identify asphyxiated newborns at risk of developing brain injury during hypothermia treatment? Am J Perinatol 32(6):555–564
Acknowledgments
We thank all the families and neonatal staff in UCLH for their support. This project was supported by EPSRC (EP/G037256/1), The Wellcome Trust (088429/Z/09/Z and 104580/Z/14/Z) and UK Department of Health’s NIHR BRC funding scheme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter‖s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter‖s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2016 The Author(s)
About this paper
Cite this paper
Mitra, S., Bale, G., Meek, J., Uria-Avellanal, C., Robertson, N.J., Tachtsidis, I. (2016). Relationship Between Cerebral Oxygenation and Metabolism During Rewarming in Newborn Infants After Therapeutic Hypothermia Following Hypoxic-Ischemic Brain Injury. In: Luo, Q., Li, L., Harrison, D., Shi, H., Bruley, D. (eds) Oxygen Transport to Tissue XXXVIII. Advances in Experimental Medicine and Biology, vol 923. Springer, Cham. https://doi.org/10.1007/978-3-319-38810-6_33
Download citation
DOI: https://doi.org/10.1007/978-3-319-38810-6_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-38808-3
Online ISBN: 978-3-319-38810-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)