Abstract
According to data from the World Health Organization, cardiovascular diseases and cancer are the two leading causes of mortality in the world [1]. Despite the immense effort to study these diseases and the constant innovation in treatment modalities, the number of deaths associated with cardiovascular diseases and cancer is predicted to increase in the coming decades [1]. From 2008 to 2030, due to population growth and population aging in many parts of the world, the number of deaths caused by cancer globally is projected to increase by 45%, corresponding to an annual increase of around four million people [1]. For cardiovascular diseases, this number is six million people [1]. In the United States, treatments for these two diseases are among the most costly and result in a disproportionate impact on low- and middleincome people. As the fight against these fatal diseases continues, it is crucial that we continue our investigation and broaden our understanding of cancer and cardiovascular diseases to innovate our prognostic and treatment approaches. Even though cardiovascular diseases and cancer are usually studied independently [2–12], there are some striking overlaps between their metabolic behaviors and therapeutic targets, suggesting the potential application of cardiovascular disease treatments for cancer therapy. More specifically, both cancer and many cardiovascular diseases have an upregulated glutaminolysis pathway, resulting in low glutamine and high glutamate circulating levels. Similar treatment modalities, such as glutaminase (GLS) inhibition and glutamine supplementation, have been identified to target glutamine metabolism in both cancer and some cardiovascular diseases. Studies have also found similarities in lipid metabolism, specifically fatty acid oxidation (FAO) and synthesis. Pharmacological inhibition of FAO and fatty acid synthesis have proven effective against many cancer types as well as specific cardiovascular conditions. Many of these treatments have been tested in clinical trials, and some have been medically prescribed to patients to treat certain diseases, such as angina pectoris [13, 14]. Other metabolic pathways, such as tryptophan catabolism and pyruvate metabolism, were also dysregulated in both diseases, making them promising treatment targets. Understanding the overlapping traits exhibited by both cancer metabolism and cardiovascular disease metabolism can give us a more holistic view of how important metabolic dysregulation is in the progression of diseases. Using established links between these illnesses, researchers can take advantage of the discoveries from one field and potentially apply them to the other. In this chapter, we highlight some promising therapeutic discoveries that can support our fight against cancer, based on common metabolic traits displayed in both cancer and cardiovascular diseases.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Glutamine metabolism
- Fatty acid oxidation
- Tryptophan catabolism
- Pyruvate dehydrogenase complex
- Cancer
- Cardiovascular diseases
-
Similarities in metabolic dysregulations between cardiovascular diseases and cancer lead to the identification of common treatment targets.
-
Alteration in circulating glutamine and glutamate levels is indicative of both cancer proliferation and cardiometabolic diseases.
-
Glutaminolysis is upregulated in cancer and pulmonary arterial hypertension.
-
Glutaminase is a treatment target for cancer, hypertension, and hyperglycemia.
-
Glutamine supplementation is implemented in the treatment of cancer to support immunoregulation and the treatment of many cardiovascular diseases.
-
Pharmacological inhibition of fatty acid oxidation is proven effective in slowing cancer progression and treating some cardiovascular diseases.
-
Inhibition of fatty acid synthesis is proven both effective in cancer treatment and protective against pulmonary hypertension.
-
Upregulated tryptophan catabolism has been linked to the progression of cardiovascular diseases and enhanced immune system evasion in cancer.
-
Pyruvate dehydrogenase complex downregulation is associated with many cardiovascular diseases and chemoresistance in cancer.
1 Introduction
According to data from the World Health Organization, cardiovascular diseases and cancer are the two leading causes of mortality in the world [1]. Despite the immense effort to study these diseases and the constant innovation in treatment modalities, the number of deaths associated with cardiovascular diseases and cancer is predicted to increase in the coming decades [1]. From 2008 to 2030, due to population growth and population aging in many parts of the world, the number of deaths caused by cancer globally is projected to increase by 45%, corresponding to an annual increase of around four million people [1]. For cardiovascular diseases, this number is six million people [1]. In the United States, treatments for these two diseases are among the most costly and result in a disproportionate impact on low- and middle-income people. As the fight against these fatal diseases continues, it is crucial that we continue our investigation and broaden our understanding of cancer and cardiovascular diseases to innovate our prognostic and treatment approaches. Even though cardiovascular diseases and cancer are usually studied independently [2,3,4,5,6,7,8,9,10,11,12], there are some striking overlaps between their metabolic behaviors and therapeutic targets, suggesting the potential application of cardiovascular disease treatments for cancer therapy. More specifically, both cancer and many cardiovascular diseases have an upregulated glutaminolysis pathway, resulting in low glutamine and high glutamate circulating levels. Similar treatment modalities, such as glutaminase (GLS) inhibition and glutamine supplementation, have been identified to target glutamine metabolism in both cancer and some cardiovascular diseases. Studies have also found similarities in lipid metabolism, specifically fatty acid oxidation (FAO) and synthesis. Pharmacological inhibition of FAO and fatty acid synthesis have proven effective against many cancer types as well as specific cardiovascular conditions. Many of these treatments have been tested in clinical trials, and some have been medically prescribed to patients to treat certain diseases, such as angina pectoris [13, 14]. Other metabolic pathways, such as tryptophan catabolism and pyruvate metabolism, were also dysregulated in both diseases, making them promising treatment targets. Understanding the overlapping traits exhibited by both cancer metabolism and cardiovascular disease metabolism can give us a more holistic view of how important metabolic dysregulation is in the progression of diseases. Using established links between these illnesses, researchers can take advantage of the discoveries from one field and potentially apply them to the other. In this chapter, we highlight some promising therapeutic discoveries that can support our fight against cancer, based on common metabolic traits displayed in both cancer and cardiovascular diseases.
2 Glutamine Metabolism as a Prognostic and Therapeutic Target in Cancer and Cardiovascular Diseases (Fig. 1)
2.1 Alterations in Circulating Glutamine and Glutamate Levels Are Indicative of Both Cancer Proliferation and Cardiometabolic Diseases
Glutamine metabolism in cancer has been extensively studied [15, 16], in which glutamine addiction has been highlighted as a signature cancer behavior. This rapid consumption of glutamine by tumors eventually results in glutamine depletion in the patient’s body and can serve as an indicator of cancer proliferation [17]. However, glutamine depletion is not always an immediate result of cancer onset. The presence of a tumor alters the host’s glutamine metabolism and creates a net flux of glutamine from other tissues and organs towards the tumor [12]. Specifically, as described in a review paper by Medina et al., glutamine synthesis is upregulated in the liver and skeletal muscles of carcinoma-bearing animals, reflected by an increase in the glutamine synthetase (GS)/glutaminase (GLS) ratio [18]. The synthesized glutamine is then secreted into the circulatory system and results in an initial elevated glutamine plasma level within the first 24 hours of tumor transplantation [18]. However, this initial increase is later overshadowed by the rapid glutamine consumption by the tumor which eventually leads to glutamine depletion in the body. The active absorption of glutamine by cancer is shown by the upregulated glutamine transportation through the plasma membrane in malignant cells [19, 20]. For cardiovascular diseases, many clinical and experimental studies have shown that the low presence of circulating glutamine is indicative of cardiometabolic diseases and hemolytic disorders [11, 21, 22]. Specifically, the low glutamine/glutamate ratio in the plasma is associated with higher blood pressure, higher circulating triglycerides, obesity, and insulin-resistant traits [11]. In contrast, a high glutamine/glutamate ratio is connected to lower risks of stroke and diabetes mellitus and is generally related to better cardiovascular health [23]. In patients with sickle cell diseases, glutamine concentration in plasma and endothelial cells has been reported to decrease significantly [24].
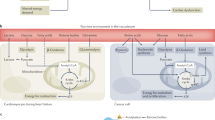
Glutamine metabolism dysregulation and corresponding therapeutic targets in cancer and cardiovascular diseases. The upregulation of glutaminolysis pathway, resulting in low glutamine and high glutamate concentrations, is observed in many cancers and cardiovascular diseases. Pharmacological inhibition of glutaminase (GLS) with BPTES and CB-839, as well as dietary glutamine supplementation, has been incorporated into the treatment of both conditions and has shown promising results
On the other hand, circulating glutamate levels have been shown to increase in breast and prostate cancer patients and reflect tumor progression to some extent [25,26,27]. The increase in glutamate levels is also associated with increased risks of cardiovascular events and decreased insulin production and sensitivity, and is positively correlated with diabetes [11, 28]. In other words, a decrease in glutamine levels and an increase in glutamate levels are generally associated with adverse health outcomes in both cancer and cardiovascular diseases. There are, however, exceptions to this trend: elevated serum glutamine levels are observed in acute myocardial ischemia patients [29].
Even though there has not been clear evidence suggesting that circulating glutamine and glutamate levels could be used for prognosis, their abnormalities have been consistently demonstrated as consequences of cancer and a variety of cardiovascular disorders. This imbalance in glutamine and glutamate levels further highlights the upregulated conversion from glutamine to glutamate, and more generally, the glutaminolysis pathway in both diseases.
2.2 Upregulation of Glutaminolysis in Cancer and Pulmonary Arterial Hypertension
The role of glutaminolysis in cancer proliferation has been well established as it contributes to tumor growth by both promoting cell proliferation and inhibiting cell death. Glutaminolysis fuels energy metabolism via the tricarboxylic acid (TCA) cycle and serves as a source of materials for biosynthesis, especially for purine and pyrimidine metabolism [15, 16]. Glutaminolysis upregulation is also associated with cancer aggressiveness, invasiveness, and metastasis [25, 30]. The upregulation of glutaminolysis has been extensively studied in many cancer types and has proven crucial for cancer development, making it a well-known target for cancer therapy. However, it is important to note that the upregulation of glutaminolysis is a trait exhibited by cancer cells, localized to the tumor, and not a signature trait exhibited by normal cells in the patient body [15, 18].
For cardiovascular diseases, glutaminolysis has been shown to increase in pulmonary endothelial cells of patients with pulmonary arterial hypertension (PAH) [11]. PAH is a potentially fatal condition caused by the abnormal proliferation of vascular cells, forming lesions that obstruct blood flow [31]. Vascular transformation, coupled with fibrosis and vasoconstriction, can lead to an increase in blood pressure and, if left untreated, can cause right ventricular failure and death [11]. Studies have shown an increased GLS1 expression and decreased glutamine levels in pulmonary endothelial cells and in myocardiocytes of the right ventricle in PAH in vivo rat model [32]. An upregulation in glutaminolysis is found in PAH patients’ right ventricular samples, suggesting that upregulated glutaminolysis is associated with PAH [32]. Furthermore, increasing glutaminolysis can result in an increased alpha-ketoglutarate level, a direct product of glutamate that helps promote the translation and stabilization of collagens, which stimulates fibrosis and worsens PAH [33]. As a result, glutaminolysis has also become a promising treatment target for PAH.
Thus, as an important energetic pathway frequently dysregulated in both cancer and cardiovascular disease metabolism, glutaminolysis has been widely investigated as a treatment target, resulting in the development of several pharmacological innovations and dietary implementations, discussed in detail in the next sections .
2.3 Glutaminase Is a Treatment Target for Cancer, Hypertension, and Hyperglycemia
GLS is an aminohydrolase enzyme that catalyzes the hydrolysis of glutamine to glutamate, which marks the initiation of glutaminolysis. There are two main types of GLS expressed in humans: GLS1, the kidney-type glutaminase, and GLS2, the liver-type glutaminase [34]. In cancer, GLS1 is expressed more often than GLS2, and thus, more frequently targeted for therapy. The most common GLS1 pharmacological inhibitors are bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) and CB-839, discussed in depth in Chap. 2, which have shown tumor suppression ability in breast cancer, leukemia, and lymphoma cells [35,36,37,38,39]. The inhibition of GLS1 expression has been extensively studied in cancer research for cancer treatment [40,41,42,43]. As discussed in the previous section, GLS1 expression is also upregulated in PAH and has been targeted for PAH treatment. Specifically, pharmacological inhibition of GLS1 using C968 or CB-839 in rats exposed to monocrotaline, a pneumotoxic liver-activated compound commonly used to induce pulmonary hypertension, significantly decreased pulmonary arteriolar and right ventricular remodeling and ameliorated pulmonary hypertension [44]. Thus, the use of GLS1-inhibiting drugs can target both cancer and PAH.
On the other hand, the role of GLS2 in cancer has been quite controversial and needs further studies to gain a comprehensive understanding [10]. A study by Martin-Rufian et al. suggests that GLS2 overexpression in cancer cells inhibits c-MYC expression [45, 46] and prevents the proliferation of several types of glioma cells [47]. In contrast, a study by Xiang et al. showed that knocking down GLS2 increases the sensitivity of cervical tumors to radiotherapy [48]. In hyperglycemia, GLS2 has been identified as a treatment target [49]. Specifically, in type 2 diabetic patients, dysregulation of glucagon causes an increase in gluconeogenesis in the liver and skeletal muscles, resulting in hyperglycemia. Inhibition of GLS2 can block glutaminolysis, decrease the hepatic metabolic flux from glutamine to glucose, decrease the initial blood glucose level, and increase insulin sensitivity [49]. Despite some attempts to study the selective inhibition of GLS1 and GLS2 [35, 49, 50], there has not been a defined pharmacological inhibition of GLS2 that is qualified for general use in research. As a promising therapeutic target, research on the role of GLS2 in cancer and cardiovascular diseases should be further expanded.
2.4 Glutamine Supplementation Is Implemented for Treatment of Cancer and a Variety of Cardiovascular Diseases
In addition to blocking glutamate production from glutamine, glutamine supplementation has also been proven effective in cardiovascular disease prevention and sickle cell disease treatment and is used in combination with chemotherapy for cancer treatment.
In cancer treatment, the use of dietary glutamine supplementation is to make up for the tumor’s “nitrogen trap” behavior that aggressively consumes dietary amino acids as well as the amino acids synthesized by the host [12]. Despite the suspicion that increasing glutamine availability may support tumor growth, a study by Austgen et al. shows that dietary supplementation of glutamine leads to no significant change in tumor growth in vivo [51]. The additional source of glutamine helps replenish glutathione (GSH) level, a crucial intracellular antioxidant, and hepatic detoxifier, in natural killer cells. Experimental data supports that glutamine supplementation impedes tumor growth by restoring the function of natural killer cells and increasing immunoregulation [52, 53]. The restored GSH level also protects patients from oxidative damages as well as increases the selectivity and decreases the cardiotoxicity and neurotoxicity effects of chemotherapeutic drugs, such as doxorubicin, methotrexate, cyclophosphamide, Eloxatin, and 5-fluorouracil, to name a few [54]. In combination with chemotherapy, supplemented glutamine can potentially help increase treatment efficacy and improve patient outcomes [55]. However, despite the promising experimental data, we still do not have enough evidence to recommend its regular use in cancer treatment. More experimental and clinical studies should be done to investigate the use of glutamine supplementation in cancer treatment.
On the other hand, the use of glutamine supplementation to protect against cardiometabolic diseases and its benefits have been widely established [11]. In diabetic patients, chronic oral administration of glutamine decreases blood glucose concentration for patients with type 1 and 2 diabetes, increases circulating insulin levels, and reduces systolic blood pressure in type 2 diabetic patients [56]. In mice, glutamine supplementations increase glucose tolerance and decrease mean arterial blood pressure [11]. For mice on a high-fat diet, studies show that glutamine intake helps lower body weight, decreases hyperglycemia, and improves insulin sensitivity [57]. In patients with coronary artery diseases, glutamine supplementation enhances myocardial repair and prevents damage to the cardiovascular system during surgical interventions or cardiac injuries [9, 58, 59]. The protective role of glutamine in ischemia-reperfusion injury is especially well investigated [60,61,62,63,64]. Glutamine reduces oxidative stress, ameliorates inflammation, and inhibits apoptosis, necrosis, and lipid peroxidation following ischemia-reperfusion. Its tissue protection effects reach a variety of organs throughout the body, including skeletal muscles, intestine, liver, kidney, brain, and heart [11]. Especially, dietary glutamine supplementation has been approved by the United States Food and Drug Administration as a treatment for sickle cell disease and is now being prescribed for patients to reduce the rate of complications [11, 65, 66]. This is a result of a successful phase 3 clinical trial, which demonstrated a significantly lower number of pain crises in patients with sickle cell disease when taking oral pharmaceutical grade glutamine. The precise mechanism of the glutamine treatment for sickle cell disease is unknown. Nevertheless, glutamine may serve as an energy source for endothelial cells and early stage reticulocytes, or premature red blood cells (RBCs) that still contain mitochondria. The established role of glutamine as a protective treatment against cardiovascular diseases reinforces the need for more clinical trials to determine its pharmacological efficacy.
Compared to cardiovascular diseases, the implementation of glutamine supplements in cancer treatment is still in its early stages of the investigation. Researchers should take advantage of the established work done on cardiovascular diseases and explore their potential integration into cancer therapy. Building upon previous knowledge can pave the way towards many groundbreaking discoveries.
3 Lipid Metabolism Plays an Important Role in Cancer Proliferation and Cardiovascular Disease Progression (Fig. 2)
3.1 Fatty Acid Oxidation in Cancer and Cardiovascular Diseases
Mitochondria fatty acid β-oxidation (FAO) involves the breakdown of energy-rich lipid molecules and plays a crucial role in the bioenergy metabolism exploited by cancer cells. The upregulation of FAO in cancer is relatively less thoroughly examined than glucose or glutamine metabolism and has been discussed in detail in Chap. 3 [67]. A variety of FAO enzymes has been shown to be overexpressed in many cancers [68]. For example, in chronic lymphocytic leukemia (CLL) cells, Liu et al. recorded a drastic increase in carnitine palmitoyltransferase 1A (CPT1A), CPT1B, and CPT2 expression [69]. High expression of CPT1A has also been shown to be indicative of poor patient outcomes in both ovarian cancer and acute myeloid leukemia (AML) [70, 71]. In prostate cancer, FAO has been identified as a dominant bioenergetics pathway and can potentially serve as a biomarker for prostate cancer diagnosis [72]. The association between FAO upregulation and metastasis has also been established in breast cancer [73, 74], colorectal cancer [75], and glioblastoma [76, 77], in which an increased FAO helps cancer cells overcome anoikis, a type of programmed cell death that occurs when anchorage-dependent cells detach from the extracellular matrix (ECM) [68]. Other studies suggest that FAO affects metastasis via cancer stem cell regulation [8, 78]. Despite the difference in its underlying mechanistic benefits towards cancer proliferation, there is an upregulation of FAO for most cancer types.
Lipid metabolism dysregulation and corresponding therapeutic targets in cancer and cardiovascular diseases. Similar metabolic dysregulations in fatty acid uptake, synthesis, and oxidation have been observed in both cancer and cardiovascular diseases, thus highlighting several common treatment targets. The “repurposing” of pharmacological inhibitors used in cardiovascular diseases to cancer therapy has shown promising experimental results. CD36 cluster of differentiation 36, FASN fatty acid synthase, CPT carnitine palmitoyltransferase, 3-KAT 3-ketoacyl-CoA thiolase
However, the alterations in FAO vary depending on the specific cardiovascular disease and the stage of the disease. The role of FAO in cardiovascular diseases is especially important, considering that it is the predominant pathway utilized by myocardiocytes for energy production [79]. In heart failure patients, many independent studies have shown an increase in the level of circulating free fatty acids and a significant decrease in the rate of FAO in the heart [80]. This downregulation of FAO is primarily due to mitochondria dysfunction and leads to an upregulated glycolysis pathway, signifying the shift back to what is known as “the fetal energy metabolism” of the failing heart. On the other hand, during ischemia-reperfusion injury, an elevated circulating level of free fatty acids increases FAO in the heart [7]. For diabetic cardiomyopathy and obese-related cardiomyopathy, there are consistent reports stating that cardiac FAO is upregulated in these conditions, along with an increase in circulating free FA and FA uptake [80]. Despite FAO upregulation, the high influx of FA overwhelms the consumption capacity, resulting in myocellular lipid accumulation and lipid toxicity in the heart, an extremely common phenomenon in these cardiomyopathy conditions [81]. The changes in FAO in these cardiovascular diseases all resulted in higher oxygen consumption per ATP molecule and an overall lower cardiac efficiency [80].
3.2 Pharmacological Inhibition of Fatty Acid Oxidation Has Proven Effective in Slowing Cancer Progression and Treating Cardiovascular Diseases
The importance of FAO on cardiovascular diseases and cancer progression has made FAO a promising target for treatment. CPT1 is one of the most common and well-investigated targets for FAO inhibition in cancer therapy. Pharmacological inhibition of CPT1 using etomoxir has shown promising anticancer results in breast cancer, leukemia, and prostate cancer [68]. In cardiovascular diseases, etomoxir was used to treat diabetes and heart failure and showed great results in vivo. However, due to toxicity effects in the liver and the heart, phase II clinical trials with etomoxir were terminated [68]. A revision of etomoxir dosage might be able to improve the clinical outcome and pave the way for effective cancer and cardiovascular disease treatment. Other CPT1 and CPT2 inhibitors such as oxfenicine, aminocarnitine, ST1326, and avocatin B, all show anticancer effects in vitro and in vivo [68].
Perhexiline is another pharmacological inhibitor of CPT1 and CPT2, currently being prescribed in Asia, Australia, and New Zealand as medicine to treat angina pectoris [13, 14], a symptom of coronary artery disease characterized by chest pain or discomfort. Studies done on chronic lymphocytic leukemia and prostate cancer cells have shown that perhexiline has a growth-inhibiting effect against cancer cells [6, 69]. Similarly used in the treatment of angina pectoris in Europe and Asia, trimetazidine is a competitive inhibitor of 3-ketoacyl-CoA thiolase (3-KAT), a component of the trifunctional protein (TFP) in FAO [68, 82]. In many studies, trimetazidine has shown positive effects in restoring heart function in hypertrophied hearts in vivo or heart failure patients [80]. In cancer, trimetazidine was also found to inhibit cell proliferation and induce apoptosis in breast cancer and glioblastoma cells in vitro [83, 84]. With the safe usage of perhexiline and trimetazidine established in cardiovascular disease, the potential clinical application of these drugs in cancer treatment should be carefully examined.
Despite the positive effect of FAO inhibition in many cardiovascular conditions, adverse cardiac consequences can arise when FAO is downregulated. Specifically, inhibited FAO can cause a buildup of lipids in cardiovascular tissues and increase lipid toxicity in the already damaged tissues. Drugs targeting FAO that lack specificity can cause damage to neighboring organs and may worsen the condition. Thus, it is crucial to take this into account when using these drugs for cancer treatment, as they might cause negative impacts on the cardiovascular system.
3.3 Inhibition of Fatty Acid Synthesis Has Proven Both Effective in Cancer Treatment and Protective Against Pulmonary Hypertension
As discussed in Chap. 3, fatty acid synthesis and fatty acid elongation are upregulated in many cancer types, including lung, breast, and bladder cancer. Its upregulation is also associated with cancer metastasis [85,86,87]. Antibody treatments targeting CD36, a fatty acid receptor, in the mouse model of human oral cancer have shown strong evidence in preventing metastasis initiation [88]. Another promising treatment targeting fatty acid synthesis for cancer therapy is TVB-2640, a fatty acid synthase (FASN) inhibitor that is currently involved in a variety of clinical trials in combination with chemotherapy [89]. FASN activity is also shown to increase in pulmonary hypertension. A study by Singh et al. shows that inhibition of FAS using siRNA in pulmonary hypertension-induced mice helps restore mitochondria function, and decrease hypertrophy, ventricular pressure, and vascular remodeling [5]. These promising results show potential for FAS inhibition to be applied to both cancer and pulmonary hypertension therapy.
4 Upregulated Tryptophan Catabolism Has Been Linked to the Progression of Cardiovascular Diseases and Enhanced Immune System Evasion in Cancer (Fig. 3)
Tryptophan is one of the nine essential amino acids that play key roles in protein synthesis and participate in the synthesis of a spectrum of crucial molecules. In mammals, the kynurenine metabolic pathway is tryptophan’s main catabolic route, resulting in 95% of peripheral tryptophan catabolism in mammals [90]. The kynurenine pathway is a complex metabolic route that is driven by the enzymes indoleamine 2,3-dioxygenase (IDO) and, to a lesser extent, tryptophan 2,3-dioxygenase (TDO). IDO has two isoenzymes, IDO1 and IDO2, with IDO1 being the primary one controlling tryptophan degradation [91]. In this pathway, tryptophan is converted to kynurenine, and the regulation is primarily associated with IDO1, the rate-limiting enzyme.
The upregulation of tryptophan catabolism is linked to the progression of cardiovascular diseases and enhanced immune system evasion in cancer. The upregulation of tryptophan 2,3-dioxygenase (TDO) and indolamine 2,3-dioxygenase (IDO) and increase in kynurenine-to-tryptophan ratio (KTR) are positively associated with risks of severe coronary events and systemic low-grade inflammation. In tumors, kynurenine binds to aryl hydrocarbon receptor (AHR), which causes the receptors to translocate into the cell’s nucleus, activating genes that help cell migration. In addition, kynurenine acts as an endogenous ligand that inhibits T cells via a variety of mechanisms to enhance immune evasion in tumors. Pharmacological inhibition of the kynurenine pathway could be applied to the treatment of cancer and cardiovascular diseases. LDL low-density lipoproteins, HDL high-density lipoproteins
In the cardiovascular system, IDO and kynurenine are known as cardiovascular relaxing factors, which bring down pressure during systemic inflammation [92]. Thus, the expression of IDO and kynurenine levels is upregulated post-inflammation and correlated with onsets of stroke events [93]. The increase in tryptophan degradation accompanied by an increased kynurenine/tryptophan ratio (KTR ) in the blood plasma is indicative of the body’s counteraction to an inflammation response, and can thus be predictive of cardiovascular events [93]. Increased KTR is also positively associated with low-density lipoproteins (LDL) and body mass index (BMI) and is negatively associated with HDL and triglycerides [94]. In individuals suffering from coronary heart disease, low tryptophan plasma concentration in combination with high KTR is prevalent [4]. The upregulation of IDO is also identified in the core of atherosclerotic plaques in humans [95].
For cancer, an elevated level of IDO expression is correlated with advanced-stage breast cancer [96]. Moreover, tryptophan degradation via the kynurenine metabolic pathway has also been linked to tumoral immune resistance [97]. Opitz et al. shows that many cancers upregulate TDO to boost tryptophan consumption [98]. When IDO and TDO are upregulated, a high level of kynurenine is synthesized, which binds to the aryl hydrocarbon receptor (AHR), inducing gene expressions that help tumor cells proliferate and metastasize. High kynurenine concentration also suppresses effector T cells, thus aiding cancer cells to evade immune responses [98]. Overexpression of IDO also occurs in different classes of immune cells, specifically antigen-presenting cells, which increases immune suppression and impedes the immune system’s ability to recognize and attack malignant cells [99].
In general, IDO upregulation in tryptophan catabolism is associated with both cardiovascular diseases and cancer and supports tumor progression via immune suppression and inflammatory tumor carcinogenesis [98]. The pharmacological administering of 1-methyl tryptophan, an IDO inhibitor, could prove to be a novel therapeutic solution for future investigation [93]. The concerted use of IDO and TDO pharmacological inhibitors could also be a potential treatment candidate for cancer therapy.
5 Pyruvate Metabolism Abnormality Is Associated with Cardiovascular Diseases and Chemoresistance in Cancer
The dysregulation of pyruvate metabolism is observed in myocardial ischemia, hypertrophy, and heart failure [3], and contributes to cancer chemoresistance [2, 100]. Pyruvate dehydrogenase complex (PDHC) is responsible for the conversion of pyruvate to acetyl-CoA, a key enzyme of aerobic cellular oxidation, and the connector between glycolysis and the TCA cycle [101, 102]. Previous studies on ischemia and reperfusion reveals a decreased flux of pyruvate through the PDHC, leading to a metabolic shift towards myocardial lactate production and a large rate of glycolysis despite high oxygen consumption [3]. PDHC activity has also been shown to decrease in hypertrophied and diabetic hearts [3]. Moreover, the upregulation of pyruvate dehydrogenase kinase (PDK), an enzyme that inactivates pyruvate dehydrogenase (the first enzyme in the PDHC), has been linked to cardiomyopathy as well as poor prognosis in patients after major cardiovascular accidents [3]. Consequentially, PDHC and PDK have become treatment targets for many cardiovascular diseases. Studies have shown that infusing pyruvate or stimulating PDHC can help recover the contractile function of the damaged heart [3]. Especially, activating PDC using the PDK inhibitor, dichloroacetate (DCA), has shown protective effects against heart failure, ischemia, and reperfusion [103] (Fig. 4).
The upregulation of PDK is also seen in many cancers and is consistently associated with invasion, metastasis, and drug resistance [104]. In bladder cancer, PDK upregulation is connected to aerobic glycolysis and chemotherapy resistance [2, 105]. A study by Woolbright et al. showed that by using DCA to inhibit PDK, bladder cancer cells showed a decrease in growth and a G0–G1 cell cycle arrest [2]. Other studies also reveal the significant role of PDK overexpression in contributing to drug resistance and the resistance to chemotherapy [105, 106]. However, despite the promising anticancer and cardioprotective effects, the use of DCA has shown many drawbacks due to its short half-life, low potency, and many toxic side effects, preventing it from being successfully incorporated into a clinical setting [107].
The shortcomings regarding DCA should not discourage researchers from identifying pyruvate metabolism as a potential treatment target for cancer and cardiovascular diseases. Promisingly, a list of small PDK-inhibitor molecules has been recently proposed, which can serve as the starting point for many investigations targeting this pathway [104]. Aside from PDK, pharmacological inhibitors of other key regulators of pyruvate metabolism and glucose oxidation should also be explored.
6 Conclusion
Altogether, many studies have pointed out similar trends in metabolic abnormalities in cancer and various cardiovascular diseases. The common metabolic dysregulations have served as overlapping treatment targets, allowing researchers and clinicians to expand their studies and treatment options. Some pharmacological inhibitors used to treat cardiovascular conditions have gone through different phases of clinical trials. However, despite also targeting problems exhibited in cancer, these drugs have not yet reached similar phases in cancer treatment. With the use of metabolomics technologies [108], researchers have continued to identify the metabolic similarities of these two diseases, paving the way for the next steps in the investigation to improve cancer therapy.
Change history
22 October 2021
After initial publication of the book, various errors were identified that needed correction. All corrections listed below have been updated within the current version.
Abbreviations
- 3-KAT:
-
3-Ketoacyl-CoA thiolase
- AHR:
-
Aryl hydrocarbon receptor
- ATP:
-
Adenosine triphosphate
- BMI:
-
Body mass index
- BPTES:
-
Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide
- CD36:
-
Cluster of differentiation 36
- CLL:
-
Chronic lymphocytic leukemia
- CPT:
-
Carnitine palmitoyltransferase
- DCA:
-
Dichloroacetate
- ECM:
-
Extracellular matrix
- FA:
-
Fatty acid
- FAO:
-
Fatty acid oxidation
- FASN:
-
Fatty acid synthase
- GLS:
-
Glutaminase
- GS:
-
Glutamine synthetase
- GSH:
-
Glutathione
- HDL:
-
High-density lipoproteins
- IDO:
-
Indoleamine 2,3-dioxygenase
- KTR:
-
Kynurenine/tryptophan ratio
- LDL:
-
Low-density lipoproteins
- PAH:
-
Pulmonary arterial hypertension
- PDHC:
-
Pyruvate dehydrogenase complex
- PDK:
-
Pyruvate dehydrogenase kinase
- siRNA:
-
Small interfering RNA
- TCA:
-
Tricarboxylic acid
- TDO:
-
Tryptophan 2,3-dioxygenase
- TFP:
-
Trifunctional protein
References
World Health Organization (2010). Chapter 1: Burden: Mortality, morbidity and risk factors. In Global status report on noncommunicable diseases. Geneva: WHO.
Woolbright, B. L., et al. (2018). The role of pyruvate dehydrogenase kinase-4 (PDK4) in bladder cancer and chemoresistance. Molecular Cancer Therapeutics, 17(9), 2004–2012.
Sun, W., et al. (2015). The role of pyruvate dehydrogenase complex in cardiovascular diseases. Life Sciences, 121, 97–103.
Wirleitner, B., et al. (2003). Immune activation and degradation of tryptophan in coronary heart disease. European Journal of Clinical Investigation, 33(7), 550–554.
Singh, N., et al. (2016). Inhibition of fatty acid synthase is protective in pulmonary hypertension. British Journal of Pharmacology, 173(12), 2030–2045.
Itkonen, H. M., et al. (2017). Lipid degradation promotes prostate cancer cell survival. Oncotarget, 8(24), 38264–38275.
Folmes, C. D., et al. (2009). High rates of residual fatty acid oxidation during mild ischemia decrease cardiac work and efficiency. Journal of Molecular and Cellular Cardiology, 47(1), 142–148.
Ito, K., et al. (2012). A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Medicine, 18(9), 1350–1358.
Chavez-Tostado, M., et al. (2017). Oral glutamine reduces myocardial damage after coronary revascularization under cardiopulmonary bypass. A randomized clinical trial. Nutrición Hospitalaria, 34(2), 277–283.
Jin, L., Alesi, G. N., & Kang, S. (2016). Glutaminolysis as a target for cancer therapy. Oncogene, 35(28), 3619–3625.
Durante, W. (2019). The emerging role of l-glutamine in cardiovascular health and disease. Nutrients, 11, 9.
Medina, M. A. (2001). Glutamine and cancer. The Journal of Nutrition, 131(9 Suppl), 2539S–2542S; discussion 2550S-1S.
Ashrafian, H., Horowitz, J. D., & Frenneaux, M. P. (2007). Perhexiline. Cardiovascular Drug Reviews, 25(1), 76–97.
Kennedy, J. A., et al. (2000). Effect of perhexiline and oxfenicine on myocardial function and metabolism during low-flow ischemia/reperfusion in the isolated rat heart. Journal of Cardiovascular Pharmacology, 36(6), 794–801.
Li, T., Copeland, C., & Le, A. (2021). Glutamine metabolism in cancer. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_2.
Le, A., et al. (2012). Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metabolism, 15(1), 110–121.
Souba, W. W. (1993). Glutamine and cancer. Annals of Surgery, 218(6), 715–728.
Medina, M. A., et al. (1992). Relevance of glutamine metabolism to tumor cell growth. Molecular and Cellular Biochemistry, 113(1), 1–15.
Espat, N. J., et al. (1995). Normalization of tumor-induced increases in hepatic amino acid transport after surgical resection. Annals of Surgery, 221(1), 50–58.
Medina, M. A., Quesada, A. R., & Nunez, I. (1991). de Castro, L-glutamine transport in native vesicles isolated from Ehrlich ascites tumor cell membranes. Journal of Bioenergetics and Biomembranes, 23(4), 689–697.
Xi, P., et al. (2011). Regulation of protein metabolism by glutamine: Implications for nutrition and health. Frontiers in Bioscience (Landmark Ed), 16, 578–597.
Cruzat, V., et al. (2018). Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients, 10, 11.
Zheng, Y., et al. (2016). Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvencion con DIeta MEDiterranea (PREDIMED) Trial. Journal of the American Heart Association, 5, 9.
Morris, C. R., et al. (2008). Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood, 111(1), 402–410.
Dornier, E., et al. (2017). Glutaminolysis drives membrane trafficking to promote invasiveness of breast cancer cells. Nature Communications, 8(1), 2255.
Budczies, J., et al. (2015). Glutamate enrichment as new diagnostic opportunity in breast cancer. International Journal of Cancer, 136(7), 1619–1628.
Koochekpour, S., et al. (2012). Serum glutamate levels correlate with Gleason score and glutamate blockade decreases proliferation, migration, and invasion and induces apoptosis in prostate cancer cells. Clinical Cancer Research, 18(21), 5888–5901.
Vangipurapu, J., et al. (2019). Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes, 68(6), 1353–1358.
Bodi, V., et al. (2012). Metabolomic profile of human myocardial ischemia by nuclear magnetic resonance spectroscopy of peripheral blood serum: A translational study based on transient coronary occlusion models. Journal of the American College of Cardiology, 59(18), 1629–1641.
Xiang, L., et al. (2019). Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death & Disease, 10(2), 40.
Chan, S. Y., & Loscalzo, J. (2008). Pathogenic mechanisms of pulmonary arterial hypertension. Journal of Molecular and Cellular Cardiology, 44(1), 14–30.
Piao, L., et al. (2013). Cardiac glutaminolysis: A maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. Journal of Molecular Medicine (Berlin), 91(10), 1185–1197.
Ge, J., et al. (2018). Glutaminolysis promotes collagen translation and stability via alpha-ketoglutarate-mediated mTOR activation and proline hydroxylation. American Journal of Respiratory Cell and Molecular Biology, 58(3), 378–390.
Saha, S. K., et al. (2019). Multiomics analysis reveals that GLS and GLS2 differentially modulate the clinical outcomes of cancer. Journal of Clinical Medicine, 8, 3.
Matre, P., et al. (2016). Inhibiting glutaminase in acute myeloid leukemia: Metabolic dependency of selected AML subtypes. Oncotarget, 7(48), 79722–79735.
Elgogary, A., et al. (2016). Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America, 113(36), E5328–E5336.
Wang, J. B., et al. (2010). Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell, 18(3), 207–219.
Robinson, M. M., et al. (2007). Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). The Biochemical Journal, 406(3), 407–414.
Gross, M. I., et al. (2014). Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Molecular Cancer Therapeutics, 13(4), 890–901.
Zimmermann, S. C., et al. (2016). Allosteric glutaminase inhibitors based on a 1,4-di(5-amino-1,3,4-thiadiazol-2-yl)butane scaffold. ACS Medicinal Chemistry Letters, 7(5), 520–524.
Xiang, Y., et al. (2015). Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. The Journal of Clinical Investigation, 125(6), 2293–2306.
Dang, C. V., et al. (2011). Therapeutic targeting of cancer cell metabolism. Journal of Molecular Medicine (Berlin), 89(3), 205–212.
Hirschey, M. D., et al. (2015). Dysregulated metabolism contributes to oncogenesis. Seminars in Cancer Biology, 35(Suppl), S129–S150.
Bertero, T., et al. (2016). Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. The Journal of Clinical Investigation, 126(9), 3313–3335.
Dang, C. V., Le, A., & Gao, P. (2009). MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clinical Cancer Research, 15(21), 6479–6483.
Le, A., & Dang, C. V. (2013). Studying Myc’s role in metabolism regulation. Methods in Molecular Biology, 1012, 213–219.
Martin-Rufian, M., et al. (2014). Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. Journal of Molecular Medicine (Berlin), 92(3), 277–290.
Xiang, L., et al. (2013). Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochimica et Biophysica Acta, 1833(12), 2996–3005.
Miller, R. A., et al. (2018). Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nature Medicine, 24(4), 518–524.
Lee, Y. Z., et al. (2014). Discovery of selective inhibitors of Glutaminase-2, which inhibit mTORC1, activate autophagy and inhibit proliferation in cancer cells. Oncotarget, 5(15), 6087–6101.
Austgen, T. R., et al. (1992). The effects of glutamine-enriched total parenteral nutrition on tumor growth and host tissues. Annals of Surgery, 215(2), 107–113.
Fahr, M. J., et al. (1994). Harry M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. JPEN Journal of Parenteral and Enteral Nutrition, 18(6), 471–476.
Yoshida, S., et al. (1995). Effect of glutamine supplementation on protein metabolism and glutathione in tumor-bearing rats. JPEN Journal of Parenteral and Enteral Nutrition, 19(6), 492–497.
Gaurav, K., et al. (2012). Glutamine: A novel approach to chemotherapy-induced toxicity. Indian Journal of Medical and Paediatric Oncology, 33(1), 13–20.
Savarese, D. M., et al. (2003). Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treatment Reviews, 29(6), 501–513.
Greenfield, J. R., et al. (2009). Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. The American Journal of Clinical Nutrition, 89(1), 106–113.
Opara, E. C., et al. (1996). L-glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. The Journal of Nutrition, 126(1), 273–279.
Khogali, S. E., et al. (2002). Is glutamine beneficial in ischemic heart disease? Nutrition, 18(2), 123–126.
Sufit, A., et al. (2012). Pharmacologically dosed oral glutamine reduces myocardial injury in patients undergoing cardiac surgery: A randomized pilot feasibility trial. JPEN Journal of Parenteral and Enteral Nutrition, 36(5), 556–561.
Zabot, G. P., et al. (2014). Glutamine prevents oxidative stress in a model of mesenteric ischemia and reperfusion. World Journal of Gastroenterology, 20(32), 11406–11414.
Kim, K. S., et al. (2013). The effect of glutamine on cerebral ischaemic injury after cardiac arrest. Resuscitation, 84(9), 1285–1290.
Prem, J. T., et al. (1999). The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. American Journal of Surgery, 178(2), 147–150.
Esposito, E., et al. (2011). Glutamine contributes to ameliorate inflammation after renal ischemia/reperfusion injury in rats. Naunyn-Schmiedeberg’s Archives of Pharmacology, 383(5), 493–508.
Stangl, R., et al. (2011). Reduction of liver ischemia-reperfusion injury via glutamine pretreatment. The Journal of Surgical Research, 166(1), 95–103.
Niihara, Y., et al. (2005). L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Blood Disorder, 5, 4.
Niihara, Y., et al. (2018). A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. The New England Journal of Medicine, 379(3), 226–235.
Park, J. K., et al. (2021). The heterogeneity of lipid metabolism in cancer. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_3.
Ma, Y., et al. (2018). Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Letters, 435, 92–100.
Liu, P. P., et al. (2016). Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline. Oncogene, 35(43), 5663–5673.
Shao, H., et al. (2016). Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer. Oncotarget, 7(4), 3832–3846.
Shi, J., et al. (2016). High expression of CPT1A predicts adverse outcomes: A potential therapeutic target for acute myeloid leukemia. eBioMedicine, 14, 55–64.
Liu, Y. (2006). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases, 9(3), 230–234.
Tan, J., & Le, A. (2021). The heterogeneity of breast cancer metabolism. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_6.
Carracedo, A., et al. (2012). A metabolic prosurvival role for PML in breast cancer. The Journal of Clinical Investigation, 122(9), 3088–3100.
Wang, Y. N., et al. (2018). CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene, 37(46), 6025–6040.
Quinones, A., & Le, A. (2021). The multifaceted glioblastoma: From genomic alterations to metabolic adaptations. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_4.
Buzzai, M., et al. (2005). The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene, 24(26), 4165–4173.
Xie, Z., et al. (2016). Inborn errors of long-chain fatty acid beta-oxidation link neural stem cell self-renewal to autism. Cell Reports, 14(5), 991–999.
Lopaschuk, G. D., et al. (2010). Myocardial fatty acid metabolism in health and disease. Physiological Reviews, 90(1), 207–258.
Fillmore, N., Mori, J., & Lopaschuk, G. D. (2014). Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. British Journal of Pharmacology, 171(8), 2080–2090.
Schulze, P. C., Drosatos, K., & Goldberg, I. J. (2016). Lipid use and misuse by the heart. Circulation Research, 118(11), 1736–1751.
Ma, Y., et al. (2020). Functional analysis of molecular and pharmacological modulators of mitochondrial fatty acid oxidation. Scientific Reports, 10(1), 1450.
Bensaad, K., et al. (2014). Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Reports, 9(1), 349–365.
Halama, A., et al. (2018). Accelerated lipid catabolism and autophagy are cancer survival mechanisms under inhibited glutaminolysis. Cancer Letters, 430, 133–147.
Jung, Y. Y., Kim, H. M., & Koo, J. S. (2015). Expression of lipid metabolism-related proteins in metastatic breast cancer. PLoS One, 10(9), e0137204.
Hua, Y., et al. (2011). Dynamic metabolic transformation in tumor invasion and metastasis in mice with LM-8 osteosarcoma cell transplantation. Journal of Proteome Research, 10(8), 3513–3521.
Luo, X., et al. (2017). Emerging roles of lipid metabolism in cancer metastasis. Molecular Cancer, 16(1), 76.
Pascual, G., et al. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature, 541(7635), 41–45.
Dean, E. J., et al. (2016). Preliminary activity in the first in human study of the first-in-class fatty acid synthase (FASN) inhibitor, TVB-2640. Journal of Clinical Oncology, 34(15_suppl), 2512–2512.
Takikawa, O. (2005). Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochemical and Biophysical Research Communications, 338(1), 12–19.
Zhai, L., et al. (2015). Molecular pathways: Targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clinical Cancer Research, 21(24), 5427–5433.
Hofmann, F. (2010). Ido brings down the pressure in systemic inflammation. Nature Medicine, 16(3), 265–267.
Mangge, H., et al. (2014). Disturbed tryptophan metabolism in cardiovascular disease. Current Medicinal Chemistry, 21(17), 1931–1937.
Pertovaara, M., et al. (2007). Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: The cardiovascular risk in Young Finns study. Clinical and Experimental Immunology, 148(1), 106–111.
Niinisalo, P., et al. (2010). Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Annals of Medicine, 42(1), 55–63.
Sakurai, K., et al. (2005). Study of indoleamine 2,3-dioxygenase expression in patients with breast cancer. Gan to Kagaku Ryoho, 32(11), 1546–1549.
Uyttenhove, C., et al. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Medicine, 9(10), 1269–1274.
Opitz, C. A., et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478(7368), 197–203.
Katz, J. B., Muller, A. J., & Prendergast, G. C. (2008). Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunological Reviews, 222, 206–221.
Le, A., Udupa, S., & Zhang, C. (2019). The metabolic interplay between cancer and other diseases. Trends Cancer, 5(12), 809–821.
Calvani, M., Reda, E., & Arrigoni-Martelli, E. (2000). Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Research in Cardiology, 95(2), 75–83.
Bose, S., Zhang, C., & Le, A. (2021). Glucose metabolism in cancer: The Warburg effect and beyond. Advances in Experimental Medicine and Biology, 1311, https://doi.org/10.1007/978-3-030-65768-0_1.
Bersin, R. M., & Stacpoole, P. W. (1997). Dichloroacetate as metabolic therapy for myocardial ischemia and failure. American Heart Journal, 134(5 Pt 1), 841–855.
Stacpoole, P. W. (2017). Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. Journal of the National Cancer Institute, 109, 11.
Sradhanjali, S., & Reddy, M. M. (2018). Inhibition of pyruvate dehydrogenase kinase as a therapeutic strategy against cancer. Current Topics in Medicinal Chemistry, 18(6), 444–453.
Lu, C. W., et al. (2011). Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. The American Journal of Pathology, 179(3), 1405–1414.
Stacpoole, P. W., et al. (1998). Clinical pharmacology and toxicology of dichloroacetate. Environmental Health Perspectives, 106(Suppl 4), 989–994.
Hoang, G., Udupa, S., & Le, A. (2019). Application of metabolomics technologies toward cancer prognosis and therapy. International Review of Cell and Molecular Biology, 347, 191–223.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Hoang, G., Nguyen, K., Le, A. (2021). Metabolic Intersection of Cancer and Cardiovascular Diseases: Opportunities for Cancer Therapy. In: Le, A. (eds) The Heterogeneity of Cancer Metabolism. Advances in Experimental Medicine and Biology, vol 1311. Springer, Cham. https://doi.org/10.1007/978-3-030-65768-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-65768-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65767-3
Online ISBN: 978-3-030-65768-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)