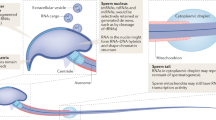
Abstract
Non-coding RNAs have recently been revealed to be intergenerational carriers of epigenetic information. Particularly the studies on the paternal epigenetic inheritance of acquired traits, metabolic disorders and psychiatric conditions, have convincingly demonstrated the involvement of sperm RNA molecules in the process. Spermatozoa contain a complex mixture of RNAs of which especially small non-coding RNAs, such as microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs) and tRNA-derived fragments (tRFs), have been shown to respond to environmental signals, exposures to toxicants, changes in diet or even early life trauma. Furthermore, recent animal studies where spermatozoal small RNAs have been injected into zygotes demostrate the capacity of RNA to transmit information about acquired conditions to offspring. Altogether, these studies highlight the central role of RNA-mediated epigenetic inheritance in the etiology of many complex diseases and therefore public health.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–64.
Anderson P, Ivanov P. tRNA fragments in human health and disease. FEBS Lett. 2014;588:4297–304.
Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9.
Aravin AAA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KFKF, Bestor T, Hannon GJGJ. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–99.
Bao J, Wu J, Schuster AS, Hennig GW, Yan W. Expression profiling reveals developmentally regulated lncRNA repertoire in the mouse male germline. Biol Reprod. 2013;89:107.
Belleannée C, Calvo E, Thimon V, Cyr DG, Légaré C, Garneau L, Sullivan R. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One. 2012;7:e34996.
Ben Maamar M, Sadler-Riggleman I, Beck D, McBirney M, Nilsson E, Klukovich R, Xie Y, Tang C, Yan W, Skinner MK. Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics. 2018;4:dvy010.
Björkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, Sipilä P. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457.
Björkgren I, Gylling H, Turunen H, Huhtaniemi I, Strauss L, Poutanen M, Sipilä P. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. 2015;29:433–42.
Brieño-Enríquez MA, García-López J, Cárdenas DB, Guibert S, Cleroux E, Děd L, de Hourcade JD, Pěknicová J, Weber M, del Mazo J. Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of MicroRNAs in primordial germ cells. PLoS One. 2015;10:e0124296.
Carmell MA, Girard A, van de Kant HJG, Bourc’his D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell. 2007;12:503–14.
Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–96.
Chalmel F, Rolland AD. Linking transcriptomics and proteomics in spermatogenesis. Reproduction. 2015;150:R149–57.
Chalmel F, Rolland AD, Niederhauser-Wiederkehr C, Chung SS, Demougin P, Gattiker A, Moore J, Patard JJ, Wolgemuth DJ, Jegou B, et al. The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci U S A. 2007;104:8346–51.
Champroux A, Cocquet J, Henry-Berger J, Drevet JR, Kocer A. A decade of exploring the mammalian sperm epigenome: paternal epigenetic and transgenerational inheritance. Front Cell Dev Biol. 2018;6:50.
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng G, Peng H, Zhang X, Zhang Y, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016a;351:397–400.
Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 2016b;17:733–43.
Conine CC, Sun F, Song L, Rivera-Pérez JA, Rando OJ. Small RNAs gained during Epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell. 2018;46:470–80.. e3
Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27.
Cortés-López M, Miura P. Emerging functions of circular RNAs. Yale J Biol Med. 2016;89:527–37.
Czech B, Hannon GJ. One loop to rule them all: the ping-pong cycle and piRNA-guided silencing. Trends Biochem Sci. 2016;41:324–37.
de Castro Barbosa T, Ingerslev LR, Alm PS, Versteyhe S, Massart J, Rasmussen M, Donkin I, Sjögren R, Mudry JM, Vetterli L, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab. 2016;5:184–97.
De Fazio S, Bartonicek N, Di Giacomo M, Abreu-Goodger C, Sankar A, Funaya C, Antony C, Moreira PN, Enright AJ, O’Carroll D. The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature. 2011;480:259–63.
de Rooij DG. The nature and dynamics of spermatogonial stem cells. Development. 2017;144:3022–30.
Deng W, Lin H. Miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell. 2002;2:819–30.
Denham J, O’Brien BJ, Harvey JT, Charchar FJ. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics. 2015;7:717–31.
Dietz DM, LaPlant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–14.
Donkin I, Barrès R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018;14:1–11.
Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, Mortensen B, Appel EVR, Jørgensen N, Kristiansen VB, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23:369–78.
Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–75.
Firlit CF, Davis JR. Morphogenesis of the residual body of the mouse testis. J Cell Sci. 1965:s3–106.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
Gapp K, Bohacek J. Epigenetic germline inheritance in mammals: looking to the past to understand the future. Genes, Brain Behav. 2018;17:e12407.
Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–9.
Gaucher J, Reynoird N, Montellier E, Boussouar F, Rousseaux S, Khochbin S. From meiosis to postmeiotic events: the secrets of histone disappearance. FEBS J. 2010;277:599–604.
Ge Z-J, Liang X-W, Guo L, Liang Q-X, Luo S-M, Wang Y-P, Wei Y-C, Han Z-M, Schatten H, Sun Q-Y. Maternal diabetes causes alterations of DNA methylation statuses of some imprinted genes in murine Oocytes1. Biol Reprod. 2013a;88:117.
Ge Z-J, Luo S-M, Lin F, Liang Q-X, Huang L, Wei Y-C, Hou Y, Han Z-M, Schatten H, Sun Q-Y. DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet–induced obesity. Environ Health Perspect. 2013b;122:159–64.
Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108.
Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202.
Gòdia M, Swanson G, Krawetz SA. A history of why fathers’ RNA matters†. Biol Reprod. 2018;99:147–59.
Goh WSSWSS, Falciatori I, Tam OHOH, Burgess R, Meikar O, Kotaja N, Hammell M, Hannon GJGJ. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev. 2015;29:1032–44.
Gou L-T, Dai P, Yang J-H, Xue Y, Hu Y-P, Zhou Y, Kang J-Y, Wang X, Li H, Hua M-M, et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2015;25:266.
Grandjean V, Fourré S, De Abreu DAF, Derieppe M-A, Remy J-J, Rassoulzadegan M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep. 2015;5:18193.
Hackett JA, Zylicz JJ, Surani MA. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 2012;28:164–74.
Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15.
Hilscher B, Hilscher W, Bülthoff-Ohnolz B, Krämer U, Birke A, Pelzer H, Gauss G. Kinetics of gametogenesis. I Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974;154:443–70.
Hirakata S, Siomi MC. piRNA biogenesis in the germline: from transcription of piRNA genomic sources to piRNA maturation. Biochim Biophys Acta - Gene Regul. Mech. 2016;1859:82–92.
Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet. 2015;16:71–84.
Hou Y-J, Zhu C-C, Duan X, Liu H-L, Wang Q, Sun S-C. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:18858.
Huypens P, Sass S, Wu M, Dyckhoff D, Tschöp M, Theis F, Marschall S, de Angelis MH, Beckers J. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet. 2016;48:497–9.
Idler RK, Yan W. Control of messenger RNA fate by RNA-binding proteins: an emphasis on mammalian spermatogenesis. J Androl. 2012;33:309–37.
Ingerslev LR, Donkin I, Fabre O, Versteyhe S, Mechta M, Pattamaprapanont P, Mortensen B, Krarup NT, Barrès R. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin Epigenetics. 2018;10:12.
Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA. The presence, role and clinical use of spermatozoal RNAs. Hum. Reprod. Update. 2013a;19:604–24.
Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA, Reproductive Medicine Network. The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013b;19:604–24.
Johnson GD, Mackie P, Jodar M, Moskovtsev S, Krawetz SA. Chromatin and extracellular vesicle associated sperm RNAs. Nucleic Acids Res. 2015;43:6847–59.
Jones RC. To store or mature spermatozoa? The primary role of the epididymis. Int J Androl. 1999;22:57–67.
Kierszenbaum AL, Tres LL. Structural and transcriptional features of the mouse spermatid genome. J Cell Biol. 1975;65:258–70.
Kleene KC, Kleene KC. Patterns, mechanisms, and functions of translation regulation in mammalian spermatogenic cells. Cytogenet Genome Res. 2003;103:217–24.
Korhonen HM, Meikar O, Yadav RP, Papaioannou MD, Romero Y, Da Ros M, Herrera PL, Toppari J, Nef S, Kotaja N. Dicer is required for haploid male germ cell differentiation in mice. PLoS One. 2011;6:e24821.
Kotaja N. MicroRNAs and spermatogenesis. Fertil Steril. 2014;101:1552–62.
Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, Diamond MP. A survey of small RNAs in human sperm. Hum Reprod. 2011;26:3401–12.
Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–49.
Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–17.
Laiho A, Kotaja N, Gyenesei A, Sironen A. Transcriptome profiling of the murine testis during the first wave of spermatogenesis. PLoS One. 2013;8:e61558.
Lane M, Zander-Fox DL, Robker RL, McPherson NO. Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends Endocrinol Metab. 2015;26:84–90.
Lehtiniemi T, Kotaja N. Germ granule-mediated RNA regulation in male germ cells. Reproduction. 2018;155:R77–91.
Li L-C. Chromatin remodeling by the small RNA machinery in mammalian cells. Epigenetics. 2014;9:45–52.
Liu W-M, Pang RTK, Chiu PCN, Wong BPC, Lao K, Lee K-F, Yeung WSB. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci U S A. 2012;109:490–4.
Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–80.
Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014;28:812–28.
Miller D, Briggs D, Snowden H, Hamlington J, Rollinson S, Lilford R, Krawetz SA. A complex population of RNAs exists in human ejaculate spermatozoa: implications for understanding molecular aspects of spermiogenesis. Gene. 1999;237:385–92.
Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–20.
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25.
Nilsson EE, Sadler-Riggleman I, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. epigenetics. 2018;4:dvy016.
Ornellas F, Carapeto PV, Mandarim-de-Lacerda CA, Aguila MB. Obese fathers lead to an altered metabolism and obesity in their children in adulthood: review of experimental and human studies. J Pediatr. 2017;93:551–9.
Öst A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–64.
Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360:772–7.
Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154.
Ostermeier GC, Goodrich RJ, Moldenhauer JS, Diamond MP, Krawetz SA. A suite of novel human spermatozoal RNAs. J. Androl. 2005;26:70–4.
Pang TY, Short AK, Bredy TW, Hannan AJ. Transgenerational paternal transmission of acquired traits: stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr Opin Behav Sci. 2017;14:140–7.
Panzeri I, Pospisilik JA. Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab. 2018;14:26–38.
Peng H, Shi J, Zhang Y, Zhang H, Liao S, Li W, Lei L, Han C, Ning L, Cao Y, et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012;22:1609–12.
Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond). 2016;130:943–86.
Radford EJ. Exploring the extent and scope of epigenetic inheritance. Nat Rev Endocrinol. 2018;14:345–55.
Rassoulzadegan M, Cuzin F. Nutrition meets heredity: a case of RNA-mediated transmission of acquired characters. Environ. Epigenetics. 2018;4:dvy006.
Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–74.
Reilly JN, McLaughlin EA, Stanger SJ, Anderson AL, Hutcheon K, Church K, Mihalas BP, Tyagi S, Holt JE, Eamens AL, et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci Rep. 2016;6:31794.
Reuter M, Berninger P, Chuma S, Shah H, Hosokawa M, Funaya C, Antony C, Sachidanandam R, Pillai RS. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature. 2011;480:264–7.
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm MicroRNA content and reprograms offspring HPA stress Axis regulation. J Neurosci. 2013;33:9003–12.
Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci. 2015;112:13699–704.
Rowold d’H E, Schulze L, Van der Auwera S, Grabe HJ. Paternal transmission of early life traumatization through epigenetics: do fathers play a role? Med. Hypotheses. 2017;109:59–64.
Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300.
Schorn AJ, Gutbrod MJ, LeBlanc C, Martienssen R. LTR-retrotransposon control by tRNA-derived small RNAs. Cell. 2017;170:61–71.. e11
Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res. 2013;41:4104–17.
Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–6.
Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev Cell. 2018;46:481–494.e6.
Short AK, Fennell KA, Perreau VM, Fox A, O’Bryan MK, Kim JH, Bredy TW, Pang TY, Hannan AJ. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl Psychiatry. 2016;6:e837.
Sipilä P, Björkgren I. Segment-specific regulation of epididymal gene expression. Reproduction. 2016;152:R91–9.
Sorscher N, Cohen LJ. Trauma in children of holocaust survivors: transgenerational effects. Am J Orthopsychiatry. 1997;67:493–500.
Soumillon M, Necsulea A, Weier M, Brawand D, Zhang X, Gu H, Barthes P, Kokkinaki M, Nef S, Gnirke A, et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep. 2013;3:2179–90.
Spadafora C. The “evolutionary field” hypothesis. Non-Mendelian transgenerational inheritance mediates diversification and evolution. Prog Biophys Mol Biol. 2018;134:27–37.
Suh N, Blelloch R. Small RNAs in early mammalian development: from gametes to gastrulation. Development. 2011;138:1653–61.
Sun J, Lin Y, Wu J. Long non-coding RNA expression profiling of mouse testis during postnatal development. PLoS One. 2013;8:e75750.
Tang F, Kaneda M, O’Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–8.
Watanabe T, Cheng E, Zhong M, Lin H. Retrotransposons and pseudogenes regulate mRNAs and lncRNAs via the piRNA pathway in the germline. Genome Res. 2015;25:368–80.
Weick E-M, Miska EA. piRNAs: from biogenesis to function. Development. 2014;141:3458–71.
Western P. Foetal germ cells: striking the balance between pluripotency and differentiation. Int J Dev Biol. 2009;53:393–409.
Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43:D146–52.
Yeshurun S, Hannan AJ. Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Mol Psychiatry. 2018;
Yuan S, Tang C, Zhang Y, Wu J, Bao J, Zheng H, Xu C, Yan W. Mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol. Open. 2015;4:212–23.
Yuan S, Schuster A, Tang C, Yu T, Ortogero N, Bao J, Zheng H, Yan W. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development. 2016;143:635–47.
Zhang P, Kang J-Y, Gou L-T, Wang J, Xue Y, Skogerboe G, Dai P, Huang D-W, Chen R, Fu X-D, et al. MIWI and piRNA-mediated cleavage of messenger RNAs in mouse testes. Cell Res. 2015;25:193–207.
Zhang X, Gao F, Fu J, Zhang P, Wang Y, Zeng X. Systematic identification and characterization of long non-coding RNAs in mouse mature sperm. PLoS One. 2017;12:e0173402.
Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, Liebers R, Zhang L, Qu Y, Qian J, et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol. 2018;20:535–40.
Zimmermann C, Stévant I, Borel C, Conne B, Pitetti J-L, Calvel P, Kaessmann H, Jégou B, Chalmel F, Nef S. Research resource: the dynamic transcriptional profile of sertoli cells during the progression of spermatogenesis. Mol Endocrinol. 2015;29:627–42.
Acknowledgements
We thank all Kotaja lab members for stimulating discussions. The research in the lab is supported by the Academy of Finland and Sigrid Jusélius Foundation. T.L. is funded by the Turku Doctoral Program in Molecular Medicine.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lehtiniemi, T., Mäkelä, M., Kotaja, N. (2020). Small Non-Coding RNAs and Epigenetic Inheritance. In: Teperino, R. (eds) Beyond Our Genes. Springer, Cham. https://doi.org/10.1007/978-3-030-35213-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-35213-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35212-7
Online ISBN: 978-3-030-35213-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)