Abstract
The ability to use different flaps and grafts is critical in the successful management of complex urethral strictures. Intimate knowledge of relevant anatomy and principles of tissue transfer are necessary to optimize outcomes. Here, we briefly review these principles and discuss a variety of techniques commonly used by reconstructive urologists depending on stricture length, stricture location, presence or absence of intact corpus spongiosum, and availability of healthy penile skin. We present our algorithms for flap or graft choice and review the pertinent literature supporting their uses.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the management of urethral stricture disease, substitution urethroplasty is a common procedure, especially in the management of longer strictures, in the setting of prior surgery, or in patients with prior hypospadias repair. Substitution tissue generally falls into two categories: flaps and grafts. Flaps refer to tissue transferred with its native blood supply intact on a pedicle. In contrast, grafts refer to tissue detached from its donor site and transferred to a recipient bed, where the establishment of a new blood supply is needed for graft “take.” This process of revascularization occurs in two steps. The first phase of graft take is imbibition, the direct diffusion of nutrients from the recipient bed across an osmotic gradient. This phase lasts approximately 24–48 hours and is followed by inosculation, which is the eventual neovascularization and capillary ingrowth between the graft and the host recipient bed. Inosculation takes several additional days.
2 Anatomy
Intimate knowledge of the blood supply of the penile skin and urethra are necessary for the successful use of tissue transfer techniques during urethral reconstruction. Additionally, an understanding of urethral anatomy allows the surgeon to choose the best tissue transfer technique depending on stricture length and location.
The normal urethra has dual arterial blood supply, which include paired bulbar arteries that enter the proximal bulbar urethra and provide a major blood supply to the corpus spongiosum. In addition, the paired dorsal arteries of the penis provide a major blood supply to the glans penis along with retrograde flow to the corpus spongiosum . Although there are also perforating vessels between the corporal bodies and the corpus spongiosum, this is a minor contribution, and therefore the normal corpus spongiosum can be mobilized along the entire penile and bulbar urethra without ischemia.
The glans and fossa navicularis represent an expansion of the corpus spongiosum with extension distal to the corporal bodies where in contrast to the penile and bulbar urethra, circumferential mobilization is not possible. In the penile and distal to mid bulbar urethra, the dorsal and ventral aspects of the corpus spongiosum have similar caliber, whereas more proximally in the bulbar urethra, the corpus spongiosum becomes considerably more robust ventrally.
The penile skin receives blood supply from branches of the superficial external pudendal artery entering at the base of the penis as posterolateral and anterolateral axial branches. They form an arterial network within the dartos fascia with an axial distribution. This allows penile skin flaps to be raised on this fascial layer with a reliable blood supply; hence they are referred to as fasciocutaneous flaps . When developing penile skin flaps, it is important to preserve the lateral and base aspects of the flaps, as the blood supply passes from lateral to medial from the base of the penile shaft. In contrast, when fasciocutaneous penile skin flaps are elevated for use in hypospadias repair or reconstruction, the remaining blood supply to the penile skin is from a superficial subdermal plexus with a random distribution (Fig. 7.1). As a consequence, if a re-do penile skin flap repair is performed, the blood supply to the flap is random and less reliable, increasing the risk of ischemic stricture recurrence in addition to penile skin deficiency. The repeated use of flaps can be associated with considerable skin loss along with stricture and this condition has often been called “hypospadias cripple ” (Fig. 7.2).
3 Substitution Material Options
There are numerous choices in graft material for use in urethral reconstruction. The use of a penile skin patch graft for one-stage urethral reconstruction was first published in 1953 as a case report by Presman [1]. In 1961, Devine began to develop a series of patch graft repairs. Then, in 1976, Devine published their 15 year experience with one-stage patch graft urethroplasty [2]. The use of grafts were popular and often preferred for substitution urethroplasty until 1980 when Duckett described the use of a transvers preputial island technique for hypospadias repair, and Quartey subsequently reported the use of a cutaneous island flap urethroplasty for the treatment of urethral strictures [3, 4]. The Quartey paper was a landmark contribution as this represented the first publication that described the fasciocutaneous blood supply of the penile skin flap, which permitted the wide mobilization of the skin for reconstruction anywhere from the external meatus to the prostatic urethra. In 1989, Schreiter described a 2-stage technique using split thickness skin grafts (STSG) , which represented an excellent new option for the treatment of recurrent strictures when sufficient penile was not available [5]. However, the use of flaps remained a favored tissue transfer method when adequate penile skin was available. This was further supported by papers describing the use of ventral penile skin flaps for reconstruction of fossa navicularis strictures with a very high success rate, and the use of circular fasciocutaneous penile flaps to repair extensive long strictures in 1-stage [6].
In 1980, Monseur described incising the urethra along the dorsal surface and fixing the opened edges of the urethra directly onto the corpora cavernosa . The urethra was then allowed to heal via secondary intention [7]. This technique was then modified by Barbagli, who combined Devine’s use of free grafts with Monseur’s dorsal urethrotomy by placing quilting a graft to the corpus cavernosa to fill the defect [7,8,9]. He then closed the edges of the dorsally incised urethra to the edges of the graft. This allowed the dorsal graft to be mechanically supported by the corpora cavernosa in addition to providing good vascular supply.
In recent years, buccal mucosal grafts (BMG) have become favored over penile skin and other graft materials [10]. This shift back to the use of graft repairs is likely due to a number of favorable characteristics of buccal mucosa. The graft is readily available, easily harvested, and can cover a large defect when bilateral grafts are taken. The donor site scar is concealed, and BMGs have been known to have low postoperative complications and good patient satisfaction [11]. Some studies have suggested that postoperative pain is improved with non-closure of the graft harvest site, further simplifying the harvest process [12]. Additionally, oral mucosa has favorable vascular characteristics, including a robust elastin-rich epithelium and a highly vascular lamina propria. This rich submucosal plexus facilities the inosculation needed for graft take. Oral mucosa is also hairless, has an epithelial surface that is well suited to a “wet” environment.
Other oral mucosal options available in the armamentarium of the reconstructive urologists include lower lip grafts and lingual mucosal grafts. Lower lip grafts have excellent surgical access but have been noted to have increased morbidity compared to buccal mucosal grafts that is likely related to long-lasting neuropathy of the mental nerve [13]. In a study of 40 patients undergoing either lower lip, inner cheek, or both grafts, Jang et al. noted that patients undergoing lower lip grafts had increased persistent postoperative discomfort, neurosensory deficits, and salivary flow changes compared to inner cheek harvest site [14]. As such, there is a tendency to favor inner check grafts over lower lip grafts when possible.
Lingual grafts are known to have similar histology compared to buccal mucosal grafts and thus have similar advantages. Additionally, lingual grafts can be harvested from one side of the tongue across the midline in continuity, allowing surgeons to achieve longer lengths than available with buccal mucosa grafts.
4 Technique Selection
Selection of the appropriate technique for a patient is a highly individualized process that depends on multiple factors. The ideal repair will depend on stricture location, length, presence or absence of healthy penile skin, and whether or not the corpus spongiosum is intact. Incorporating all of these factors is a complex process, but the proper selection of tissue transfer technique is crucial for success. Our aim is to provide a logical, easily comprehensible approach to the appropriate technique selection in urethral reconstruction.
5 Tissue Transfer Techniques to the Glans and Fossa Navicularis
An algorithmic approach to glans and fossa navicularis strictures is outlined in Fig. 7.3. When strictures are truly limited to the glans penis alone (meatal stenosis), a simple or extended meatotomy is the minimally invasive procedure of choice [15]. If the stricture extends further either into the fossa navicularis or is limited to the fossa navicularis, an extended meatotomy continues to represent a simple definitive treatment option that is generally curative as opposed to periodic dilations, which represent a management approach. However, as the stricture extends proximal to the meatus, the extension of the meatotomy will be associated with an elongation and ventral displacement of the urethral opening. A one-stage flap repair is a good option, provided the skin is healthy and adequate and there has been no prior flap surgery that would compromise the use of penile skin. In these cases, we prefer to use a one-stage ventral transverse island fasciocutaneous penile flap as described by Jordan [16]. After a ventral incision through the stricture, a transverse island penile skin flap is elevated on a broad dartos pedicle and inverted onto the defect prior to closure of the glans wings (Fig. 7.4). Due to the relative laxity of penile skin, this flap can be used even in circumcised patients. This procedure has excellent long-term success rate with a reported stricture-free rate of 100% among patients who had no lichen sclerosis (LS) also known as Balanitis Xerotica Obliterans (BXO) , but a 50% recurrence rate in those with LS [17]. This is not surprising. Bracka and Mundy both nicely demonstrated that the use of penile skin in patients with LS for urethroplasty is associated with a very high late recurrence rate, and this report caused a shift away from the use of flaps in favor of graft repairs when the skin is not healthy due to a diagnosis of LS [18, 19]. Therefore, in patients with LS or an unreliable penile skin blood supply due to prior flap surgery, our preference is staged graft repair (Fig. 7.5). While our early experience favored the use of STSG, we now exclusively use buccal mucosa grafts for these staged repairs.
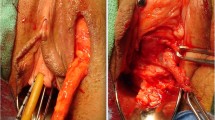
Staged urethroplasty with buccal mucosal graft performed for a patient with a fossa and penile urethral stricture. (a) An extended meatotomy is made through the stricture. (b) BMG quilted on both sides of the opened urethral plate. (c) The ventral flap has been rotated as a ventral onlay to cover the defect. (d) Immediate appearance after closure
Staged urethroplasty with buccal mucosal graft performed for a patient with a fossa and penile urethral stricture. (a) Demonstration of inadequate penile skin. (b) BMG quilted on both sides of the opened urethral plate. (c) The urethral plate after 4–6 months of healing from the first stage procedure. (d) Tubularization of the new urethral plate. (e) Appearance immediately postoperatively. (f) Appearance 3 weeks postoperatively
There have been recent reports of one-stage buccal graft repairs of glans or fossa navicularis strictures BMG [20, 21]. Chowdury et al. described the use of ventral onlay buccal mucosal grafts using the glans wings as graft beds [20]. Certainly it would be advantageous to repair these strictures with a 1-stage approach. However, we do not believe that these techniques in our hands obtain the same caliber of patency (24–30 French) that can be achieved with ventral flaps (when appropriate) or staged repairs.
Distal strictures in patients with either LS or prior hypospadias repairs represent a challenge to reconstructive urologists where careful selection of repair type and tissue substitution choice can significantly affect long term outcomes. Most importantly, it should be emphasized that the priority of repair is relief of obstruction. Thus, the caliber of the repair, regardless of technique, should not be compromised by aggressively attempting to bring the meatus all the way to the tip of the glans.
6 Tissue Transfer to the Penile Urethra and Bulbar Urethra
The repair of penile and bulbar urethral strictures that are not amenable to anastomotic urethroplasty can be performed in one-stage with flaps or grafts, or staged procedures. Decision making regarding repair type depends largely on whether the corpus spongiosum is intact and whether there is sufficient penile skin, as summarized in the algorithm in Fig. 7.6.
6.1 When the Corpus Spongiosum is Intact- Penile and/or Bulbar Urethra
In patients with intact corpus spongiosum without LS, one-staged procedures are preferred [22]. Overall, the consensus of the current literature provides evidence that buccal mucosa grafts are superior to flaps for 1 stage penile and bulbar stricture repair. Moreover, the use of dorsal grafts preserves the corpus spongiosum ventrally and laterally, essentially eliminating the risk of urethrocutaneous fistula formation, and the support of the urethra by the corporal bodies dorsally and the corpus spongiosum laterally and ventrally prevents the development of diverticular change, and may reduce the risk of false passage should catheterization be indicated as some future time. Although we favor dorsal grafting in both the penile and bulbar urethra, some authors prefer a ventral approach, as it limits the need for urethral mobilization and allows preservation of cavernosal-spongiosal perforating arteries [23, 24]. Additionally, the ventral approach is often considered less technically challenging with shorter operative times [24, 25].
There are some disadvantages associated with ventral placement of BMG. Since the ventral aspect of the bulbar urethra is more robust and highly vascular, some surgeons note increased blood loss at time of surgery with a ventral urethrotomy [26]. Additionally, there is a risk of urethral sacculation or fistula development due to decreased support of the urethra compared to dorsal graft placement.
The dorsal onlay approach offers several advantages over the ventral onlay approach . The graft can be spread fixated to the corpora cavernosa, which is a consistently reliable graft recipient bed. Additionally, the spread-fixation, which cannot be accomplished with the ventral approach, maximizes the surface area of the graft in direct contact with the vascular bed [22]. This optimizes the conditions for graft take and provides up to 2+ cm widening of the urethral plate. It is unclear from the literature whether one technique is superior to the other. No randomized controlled trials comparing the dorsal and ventral onlay BMG urethroplasty exist.
Numerous modifications to the original dorsal onlay graft have been described. For example, in 2001, Asopa et al. described the use of a ventral and dorsal urethrotomy with subsequent placement of an elliptical dorsal inlay graft via the exposure achieved from the ventral urethral incusion prior to ventral urethrotomy closure for anterior stricture repair [27]. It allows for spread-fixation of the BMG dorsally with a reliable vascular bed while maintaining advantages of ventral onlay grafts by minimizing urethral mobilization and preservation perforating vessels. However, this technique requires the urethra to be incised both dorally and ventrally.
Kulkarni et al. modified the dorsal onlay procedure in cases of panurethral stricture disease secondary to LS by using a perineal approach, inverting the penis, and extending the dorsal onlay into the fossa navicularis to repair these long strictures in 1-stage [21].
Each of these options has advantages and disadvantages that can be applied in certain clinical scenarios. Until a higher level of evidence emerges, it is our practice to preferentially use the dorsal onlay approach for the advantages mentioned above.
6.2 When the Corpus Spongiosum is Not Intact
In some scenarios, the corpus spongiosum is not intact . In patients with hypospadias, the distal corpous spongiosum is often poorly developed and not amenable to circumferential mobilization. Thus, in these cases, a one-stage graft is not recommended [22]. In addition, when there has been prior dorsal graft reconstruction, circumferential mobilization of the corpus spongiosum would detach the buccal graft from its recipient bed blood supply. When there has been a prior flap, circumferential mobilization would likely devascularize the flap. In these cases, if the penile skin is not affected by LS and there has been no prior flap surgery, a fasciocutaneous penile then a skin flap is an option for one-stage repair. When there is a paucity of healthy skin (patients with prior hypospadias or LS), then use of a penile skin flap is contraindicated.
Historically, skin grafts were initially used for staged procedures with a success rate of approximately 50% [28]. With the increased use of BMG graft, the success rate has increased to 80% [29]. Similarly, our presented but currently unpublished institutional data noted an overall success rate of 81.6% in our series of 49 patients undergoing staged urethroplasty with mean follow-up of 32 months. During subset analysis, patients treated exclusively with BMG had higher success rate compared to those who had STSG (100% vs. 64%, p < 0.01). Thus, when possible, we preferentially use BMG during staged repairs.
7 Special Situations
7.1 Augmented Anastomosis
In some cases, there is a segment of obliterated or near-obliterated urethra associated with significant spongiofibrosis. If the stricture is too long to be amenable to excisional repair, an augmented anastomosis can be considered. It is especially favorable if there is wider stricture adjacent to a shorter obliterative segment. After excision of the obliterated stricture, a dorsal mucosal anastomosis with a placement of a flap or graft to augment the ventral urethral wall can be performed. The graft can also be placed as a dorsal onlay with re-anastomosis of the ventral urethral mucosa [30, 31]. More contemporary series have reinforced the efficacy of the augmented anastomotic urethroplasty with success rates >90%, though studies have heterogeneous follow up and definitions of success [32,33,34].
7.2 Graft/Flap Combination
In patients with an obliterative segment and/or significant spongiofibrosis , strictures can be managed with the combined use of dorsal onlay BMG to augment or replace the inadequate urethral plate, followed by a penile skin flap ventral onlay reconstruction (Fig. 7.7). This was first described by Morey in 2001 in four patients with long penile urethral strictures who required BMG (n = 3) or cadaveric dermal graft (n = 1) to augment or replace the urethral plate [35]. Patients then underwent simultaneous transverse penile island ventral onlay skin flap. This allowed for repair in a single stage while avoiding the poor results of single-stage tubularized flaps or grafts for circumferential tissue transfer reconstruction. A larger series was described by Gelman et al. in 2011 with 12 patients with distal urethral strictures who underwent combined dorsal onlay BMG with ventral onlay penile skin flap [36]. Most patients had hypospadias or recurrent strictures that increased complexity of stricture repair, but a 92% success rate was observed as defined by wide patency at 4-month cystoscopy with subsequent mean follow-up of 39 months.
Graft/flap combination . (a) The obliterated urethra is incised proximally until healthy, widely patent urethral lumen is encountered. (b) The BMG is spread fixated to the corpora cavernosa. Then a penile skin flap is rotated ventrally onto the graft to create a new lumen. In cases where there is a deficiency of urethra within the fossa and a lack of a groove within the glans penis, a defect is created and the BMG is extended into the glans
The combined use of flap and graft can also be used for long panurethral strictures that are too long to be repaired with BMG, even if bilateral grafts are harvested. Berglund and Angermeier described a series of 18 patients who underwent a combined graft/flap repair for complex strictures with a mean length of 15.1 cm [37]. They were able to achieve an 83% success rate at last follow-up.
7.3 Combined Dorsal/Ventral Buccal Grafting
This was first described via a ventral approach by Palminteri et al. in 2008 when he used the Asopa technique to make a ventral urethrotomy with placement of dorsal onlay BMG into the incised dorsal urethral plate and subsequent placed additional graft as a ventral onlay [38]. He noted very good success rate of 89.6% at mean follow-up of 22 months in 48 men. Further long-term follow up of 166 patients undergoing dorsal plus ventral oral graft (DVOG) showed 89.8% success at median follow-up of 47 months, with 90% of failures occurring in the first 5 years [39]. Additionally, postoperative sexual function as determined by validated questionnaire noted preservation of sexual function in all patients with preoperative sexual function [40].
However, Palminteri’s technique relies on sufficient urethral plate to perform an Asopa procedure . Some surgeons have suggested that a urethral plate <1 cm should be a contraindication to performing an Asopa, and thus, a Palminteri DVOG [41]. In 2014, Gelman et al. published a technique targeted towards obliterative urethral strictures [42]. He presented a series of 18 patients with obliterative or near-obliterative bulbar urethral strictures who underwent a combined ventral and dorsal buccal grafting for a single stage repair via a dorsal approach. The technique, currently referred to as a double faced buccal urethroplasty , differed in that the urethra was mobilized and a dorsal urethrotomy was made without ventral incision of the urethra, thus preserving the continuity of blood supply within the spongy tissue. BMG was then quilted dorsally onto the corporal bodies. After excision of obliterated urethral scar, additionally BMG was quilted ventrally through the dorsally incised urethtomy onto the corpus spongiosum in continuity with the distal and proximally spatulated urethra. The dorsal and ventral BMG segments were then approximated, completing the repair (Fig. 7.8).
Dorsal and ventral buccal graft combination . (a) The urethra is mobilized and incised dorsally. Fibrotic scar is removed until healthy ventral spongiosum is exposed. (b) BMG is spread fixated ventrally to the spongiosum where the obliterated segment was located in continuity with the native mucosa. Additional BMG is then dorsally quilted to the corpora cavernosa before the edges are reanastamosed for tubularization
Some advantages of the repair described by Gelman et al. are that the robust ventral spongiosum is left intact. Additionally, dorsal onlay grafts can be spread-fixed to the corpus cavernosum, which are a reliable vascular bed for the graft. With mean follow-up of 50 months, patients undergoing this repair had a 94% success rate [42].
8 Summary
Tissue transfer techniques with flaps and grafts serve as an indispensable tool in the armamentarium of reconstructive urologists. Familiarity with a large variety of techniques and tissue transfer types allow urologists to tailor their repair based on stricture location, stricture length, amount of viable native mucosa, and whether the corpus spongiosum is intact. While there is no clear consensus on a single superior method based on the literature, all techniques mentioned are successful in the appropriately selected patient.
Key Summary Points
-
Over time, there has been a transition from penile skin grafting to penile flaps, then most recently to the use of buccal mucosal grafting.
-
The ideal technique will depend on stricture location, length, presence or absence of healthy penile skin, and the status of the corpus spongiosum.
-
For distal meatal and fossa navicularis strictures, staged repairs with graft are preferred over flaps when lichen sclerosus is present.
-
Multiple techniques exist for placement of buccal graft in penile and bulbar strictures, each with its own advantages and disadvantages.
-
While there is no clear consensus on a single superior method based on the literature, all techniques mentioned in this chapter are reported to be successful in the appropriately selected patient.
Change history
16 December 2021
https://doi.org/10.1007/978-3-030-21447-0_7
References
Presman D, Greenfield D. Reconstruction of the perineal urethra with a free full-thickness skin graft from the prepuce. J Urol. 1953;69(5):677–80.
Devine PC, Fallon B, Devine CJ Jr. Free full thickness skin graft urethroplasty. J Urol. 1976;116(4):444–6.
Duckett JW Jr. Transverse preputial island flap technique for repair of severe hypospadias. Urol Clin North Am. 1980;7(2):423–30.
Quartey JK. One-stage penile/preputial island flap urethroplasty for urethral stricture. J Urol. 1985;134(3):474–5.
Schreiter F, Noll F. Mesh graft urethroplasty using split thickness skin graft or foreskin. J Urol. 1989;142(5):1223–6.
McAninch JW. Reconstruction of extensive urethral strictures: circular fasciocutaneous penile flap. J Urol. 1993;149(3):488–91.
Monseur J. [Widening of the urethra using the supra-urethral layer (author’s transl)]. J Urol (Paris). 1980;86(6):439–49.
Barbagli G, Selli C, Tosto A, Palminteri E. Dorsal free graft urethroplasty. J Urol. 1996;155(1):123–6.
Devine PC, Wendelken JR, Devine CJ Jr. Free full thickness skin graft urethroplasty: current technique. J Urol. 1979;121(3):282–5.
Blaschko SD, Harris CR, Zaid UB, Gaither T, Chu C, Alwaal A, et al. Trends, utilization, and immediate perioperative complications of urethroplasty in the United States: data from the national inpatient sample 2000–2010. Urology. 2015;85(5):1190–4.
Barbagli G, Vallasciani S, Romano G, Fabbri F, Guazzoni G, Lazzeri M. Morbidity of oral mucosa graft harvesting from a single cheek. Eur Urol. 2010;58(1):33–41.
Wood DN, Allen SE, Andrich DE, Greenwell TJ, Mundy AR. The morbidity of buccal mucosal graft harvest for urethroplasty and the effect of nonclosure of the graft harvest site on postoperative pain. J Urol. 2004;172(2):580–3.
Kamp S, Knoll T, Osman M, Hacker A, Michel MS, Alken P. Donor-site morbidity in buccal mucosa urethroplasty: lower lip or inner cheek? BJU Int. 2005;96(4):619–23.
Jang TL, Erickson B, Medendorp A, Gonzalez CM. Comparison of donor site intraoral morbidity after mucosal graft harvesting for urethral reconstruction. Urology. 2005;66(4):716–20.
Morey AF, Lin HC, DeRosa CA, Griffith BC. Fossa navicularis reconstruction: impact of stricture length on outcomes and assessment of extended meatotomy (first stage Johanson) maneuver. J Urol. 2007;177(1):184–7; discussion 7.
Jordan GH. Reconstruction of the fossa navicularis. J Urol. 1987;138(1):102–4.
Virasoro R, Eltahawy EA, Jordan GH. Long-term follow-up for reconstruction of strictures of the fossa navicularis with a single technique. BJU Int. 2007;100(5):1143–5.
Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterance. Br J Urol. 1998;81(5):735–7.
Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int. 2000;86(4):459–65.
Chowdhury PS, Nayak P, Mallick S, Gurumurthy S, David D, Mossadeq A. Single stage ventral onlay buccal mucosal graft urethroplasty for navicular fossa strictures. Indian J Urol. 2014;30(1):17–22.
Kulkarni SB, Joshi PM, Venkatesan K. Management of panurethral stricture disease in India. J Urol. 2012;188(3):824–30.
Wisenbaugh ES, Gelman J. The use of flaps and grafts in the treatment of urethral stricture disease. Adv Urol. 2015;2015:979868.
Figler BD, Malaeb BS, Dy GW, Voelzke BB, Wessells H. Impact of graft position on failure of single-stage bulbar urethroplasties with buccal mucosa graft. Urology. 2013;82(5):1166–70.
Palminteri E, Berdondini E, Di Pierro GB. The advantages of the ventral approach to bulbar urethroplasty. Arab J Urol. 2013;11(4):350–4.
Armenakas NA. Long-term outcome of ventral buccal mucosal grafts for anterior urethral strictures. AUA News. 2004;9:2.
Brandes SB. Editorial comment: oral mucosal graft urethroplasty. In: Brandes SB, Morey AF, editors. Advanced male urethral and genital reconstructive surgery. 2. New York: Humana Press; 2014. p. 193–4.
Asopa HS, Garg M, Singhal GG, Singh L, Asopa J, Nischal A. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology. 2001;58(5):657–9.
Mori RL, Angermeier KW. Staged urethroplasty in the management of complex anterior urethral stricture disease. Transl Androl Urol. 2015;4(1):29–34.
Barbagli G, De Angelis M, Palminteri E, Lazzeri M. Failed hypospadias repair presenting in adults. Eur Urol. 2006;49(5):887–94; discussion 95.
Turner-Warwick R. Principles of urethral reconstructions. In: Webster GD, Kirby R, King L, editors. Reconstructive urology. 2. Boston: Blackwell Scientific; 1993. p. 609–42.
Guralnick ML, Webster GD. The augmented anastomotic urethroplasty: indications and outcome in 29 patients. J Urol. 2001;165(5):1496–501.
Hoy NY, Kinnaird A, Rourke KF. Expanded use of a dorsal onlay augmented anastomotic urethroplasty with buccal mucosa for long segment bulbar urethral strictures: analysis of outcomes and complications. Urology. 2013;81(6):1357–61.
El-Kassaby AW, El-Zayat TM, Azazy S, Osman T. One-stage repair of long bulbar urethral strictures using augmented Russell dorsal strip anastomosis: outcome of 234 cases. Eur Urol. 2008;53(2):420–4.
Abouassaly R, Angermeier KW. Augmented anastomotic urethroplasty. J Urol. 2007;177(6):2211–5; discussion 5–6.
Morey AF. Urethral plate salvage with dorsal graft promotes successful penile flap onlay reconstruction of severe pendulous strictures. J Urol. 2001;166(4):1376–8.
Gelman J, Sohn W. 1-stage repair of obliterative distal urethral strictures with buccal graft urethral plate reconstruction and simultaneous onlay penile skin flap. J Urol. 2011;186(3):935–8.
Berglund RK, Angermeier KW. Combined buccal mucosa graft and genital skin flap for reconstruction of extensive anterior urethral strictures. Urology. 2006;68(4):707–10; discussion 10.
Palminteri E, Manzoni G, Berdondini E, Di Fiore F, Testa G, Poluzzi M, et al. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol. 2008;53(1):81–9.
Palminteri E, Lumen N, Berdondini E, Di Pierro GB, Cucchiarale G, Tenti G, et al. Two-sided dorsal plus ventral oral graft bulbar urethroplasty: long-term results and predictive factors. Urology. 2015;85(4):942–7.
Palminteri E, Berdondini E, Shokeir AA, Iannotta L, Gentile V, Sciarra A. Two-sided bulbar urethroplasty using dorsal plus ventral oral graft: urinary and sexual outcomes of a new technique. J Urol. 2011;185(5):1766–71.
Marshall SD, Raup VT, Brandes SB. Dorsal inlay buccal mucosal graft (Asopa) urethroplasty for anterior urethral stricture. Transl Androl Urol. 2015;4(1):10–5.
Gelman J, Siegel JA. Ventral and dorsal buccal grafting for 1-stage repair of complex anterior urethral strictures. Urology. 2014;83(6):1418–22.
Conflicts of Interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Gelman, J., Furr, J. (2020). Tissue Transfer Techniques in the Management of Urethral Stricture Disease: Flaps and Grafts. In: Martins, F.E., Kulkarni, S.B., Köhler, T.S. (eds) Textbook of Male Genitourethral Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-030-21447-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-21447-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21446-3
Online ISBN: 978-3-030-21447-0
eBook Packages: MedicineMedicine (R0)