Abstract
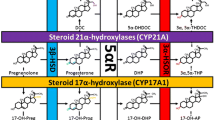
5α-reductase is a nuclear membrane-bound NADPH-dependent δ-3-ketosteroid 5α-oxidoreductase. It is found in androgen-sensitive tissues, and catalyzes the conversion of testosterone to dihydrotestosterone (DHT). Two known isoenzymes of 5α-reductase have been identified: Type 1 predominates in peripheral tissues such as the skin and liver, and Type 2 is predominant in the prostate. Finasteride is a specific inhibitor of the Type 2 isoenzyme, and effectively blocks the Type 2-mediated conversion of testosterone to DHT, but does not inhibit the binding of DHT to the androgen receptor (AR) (1).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
US product circular Medical Economics Company, Inc., for PROSCAR® (finasteride 5 mg tablets), in Physicians’ Desk Reference 53rd ed., Montvale, NJ, 1999, pp.1880–1883.
Osterling JE. Prostate-specific antigen: a critical assessment of the most useful marker for adenocarcinoma of the prostate. J Urol 1991;145:907–923.
Andriole G, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-Term Efficacy and Safety Study. Urology 1998;52:195–201.
Ohtawa M, Morikawa H, Shimazaki J. Pharmacokinetics and biochemical efficacy after single and multiple oral administration of N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17 β-carboxamide, a new type of specific competitive inhibitor of testosterone 5α-reductase, in volunteers. Eur J Drug Metab Pharmacokinet 1991;16,15–21.
Carlin JR, Höglund P, Eriksson LO, et al. Disposition and pharmacokinetics of [14C] finasteride after oral administration in humans. Drus Metab Dispos 1992;20,148–155.
Huskey SW, Dean DC, Miller RR, et al. Identification of human cytochrome P450 isozymes responsible for the in vitro oxidative metabolism of finasteride. Drug Metal Dispos 1995;23,1126–1135.
Gormley GJ, Stoner E, Rittmaster RS, et al. Effects of finasteride (MK-906), a 5α-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab 1990;70,1136–1141.
Stoner E, the Finasteride Study Group. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology 1994;43,284–294.
Rittmaster RS, Lemay A, Zwicker H, et al. Effects of finasteride, a 5α-reductase inhibitor, on serum gonadotropins in normal men. J Clin Endocrinol Metab 1992;75,484–488.
Tenover JS, Zeitner ME, Plymate SR. Effects of 24-week administration of a 5α-reductase inhibitor (MK-906) on serum levels of testosterone (T), free T, and gonadotropins in men. Proceedings of 71st Annual Meeting of the Endocrine Society; Program Abstr Endoc Soc Annu Meet 1989;71:abst. S83.
Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000;50(1):7–33.
Gaddipati J, Ahmed T, Friedland M. Prostatic and bladder cancer in the elderly. Clin Geriatr Med 1987;3,649–667.
Smith PH, Armitage TG. Immediate vs deferred treatment for early prostatic cancer. Postgrad Med J 1987;63,1055–1060.
Isaacs JT, Coffey DS. Adaptation vs selection as the mech-anism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res 1981;41,5070–5075.
Huggins C, Hodges CV. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941;1,293–297.
Huggins C, Stevens RE, Hodges CV. II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1943;43,209–223.
Melamed A. Current concepts in the treatment of prostate cancer. Clin Pharm 1987;21,247–253.
Smith J. New methods of endocrine management of prostatic cancer. J Urol 1987;137,1–10.
Trump DL, Waldstreicher JA, Kolvenbag G, et al. Androgen antagonists: potential role in prostate cancer prevention. Urology 2001;57 (Suppl 4A):64–67.
Ross RK, Bernstein L, Judd H, et al. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst 1976;76:45.
Bruun E, Frandsen H, Nielsen K, et al. Dihydrotestosterone measured in core biopsies from prostatic tissues. Am J Clin Oncol 1988 (Suppl 2):S27–S29.
Habib FK, Bissas A, Neill WA, et al. Flow cytometric analysis of cellular DNA in human prostate cancer: relationship to 5α-reductase activity of the tissue. Urol Res 1989;17:239–243.
Muir C, Waterhouse J, Mack T, et al. Cancer Incidence in Five Continents, Vol. V. IARC, Lyon, France, 1987.
SEER: Cancer Incidence and Mortality in the United States, 1973–1981. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, Bethesda, MD, 1984.
Ross RK, Bernstein L, Lobo RA, et al. Evidence for reduced 5α-reductase activity in Japanese compared to US Caucasian and African-American males: implications for prostate cancer risk. Lancet 1992;339:887–889.
Bologna M, Muzi P, Biordi L et al. Antiandrogens and 5α-reductase inhibition of the proliferation rate in PC3 and DU 145 human prostatic cancer cell lines. Curr Ther Res 1992;51:799–813.
Kadoham N, Karr JP, Murphy GP, Sandberg AA. Selective inhibition of prostatic tumor 5α-reductase by a 5-methyl-4-aza-steroid. Cancer Res 1984;44:4947–4954.
Petrow V, Padilla GM, Mukherji S, Marts SA. Endocrine dependence of prostatic cancer upon dihydrotestosterone and not upon testosterone. J Pharm Pharmacol 1984;36:352–353.
McConnell JD, Wilson JD, George FW, et al. Finasteride, an inhibitor of 5α-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. Clin Endocrinol Metab 1992;74,505–508.
Presti JC Jr, Fair WR, Andriole G et al. Multicenter, randomized, double blind, placebo controlled study to investigate the effect of finasteride (MK-906) on stage D prostate cancer. J Urol 1992;148:11,201–11,204.
Nacey JN, Meffan PJ, Delahunt B. The effect of finasteride on prostate volume, urinary flow rate and symptom score in men with benign prostatic hyperplasia. N Z Med J 1993;106:109–110.
Oesterling JE, Roy J, Agha A, et al. Finasteride PSA study group. Biologic variability of prostate-specific antigen and its usefulness as a marker for prostate cancer: effects of finasteride. Urology 1997;50:13–18.
Andriole G, Block N, Boake R, et al. Two years of treatment with finasteride after radical prostatectomy. J Urol 1994;151:435A.
Andriole G, Lieber M, Smith J, et al. Treatment with finasteride following radical prostatectomy for prostate cancer. Urology 1995;45:491–497.
Jones CD, Audia JE, Lawhorn DE, et al. Nonsteroidal inhibitors of human Type I steroid 5α-reductase. J Med Chem 1993;36:421–423.
Hirsch KS, Jones CD, Audia JE, et al. LY191704: a selective, nonsteroidal inhibitor of human steriod 5α-reductase Type 1. Proc Natl Acad Sci USA 1993;90:5277–5281.
Ellsworth K, Azzolina B, Baginsky W, et al. MK386: a potent, selective inhibitor of the human Type 1 5α-reductase. J Steroid Biochem Mol Biol 1996;58:377–384.
Schwartz JI, Tanaka WK, Wang DZ, et al. MK-386, an inhibitor of 5α-reductase Type 1, reduces dihydrotestosterone concentrations in serum and sebum without affecting dihydrotestosterone concentrations in semen. J Clin Endocrinol Metab 1997;82:1373–1377.
Harris G, Azzolina B, Baginsky W, et al. Identification and selective inhibition of an isozyme of steroid 5α-reductase in human scalp. Proc Nall Acad Sci USA 1992;89:10,787–10,791.
Russell DW, Wilson JD. Steroid 5α-reductase two genes/two enzymes. Annu Rev Biochem 1994;63:25–61.
Thigpen AE, Silver RI, Guileyardo JM, et al. Tissue distribution and ontogeny of steroid 5α-reductase isozyme expression. J Clin Investig 1993;92:903–910.
Thiboutot D, Harris G, Iles V, et al. Activity of the Type 1 5α-reductase exhibits regional difference in isolated sebaceous glands and whole skin. J Invest Dermatol 1995;105:209–214.
Pelletier G, Luu-The V, Huang XF, Lapointe H, Labrie F. Localization by the situ hybridization of steroid 5α-reductase isozyme gene expression in the human prostate and preputial skin. J Urol 1998;160:577–582.
Habib FK, Ross M, Bayne CW, et al. The localization and expression of 5α-reductase Type I and Type II mRNAs in human hyperplastic prostate and in prostate primary cultures. J Endocrinol 1998;156:509–517.
Iehlé C, Radvanyi F, Diez de Medina, SG, et al. Differences in steroid 5α-reductase iso-enzymes expression between normal and pathological human prostate tissue. J Steroid Biochem Mol Biol 1999;68:189–195.
Bruchovsky N, Sadar MD, Akakura K, et al. Characterization of 5α-reductase gene expression in stroma and epithelium of human prostate. J Steroid Biochem 1996;59:397–404.
Span PN, Benraad TJ, Sweep CGJ, Smals AGH. Kinetic analysis of steroid 5α-reductase activity of neutral pH in benign prostatic hyperplastic tissue: evidence for Type I isozyme activity in the human prostate. J Steroid Biochem Mol Biol 1996;57:103–110.
Smith CM, Ballard SA, Worman N, et al. 5α-reductase expression by prostate cancer cell lines and benign prostatic hyperplasia in vitro. J Clin Endocrinol Metab 1996;81:1361–1366.
Negri-Cesi P, Poletti A, Colciago A, et al. Presence of 5α-reductase isozymes and aromatase in human prostate cancer cells and in benign prostate hyperplastic tissue. Prostate 1998;34:283–291.
Silver RI, Wiley EL, Davis DL, et al. Expression and regulation of steroid 5α-reductase 2 in prostate disease. J Urol 1994;152:433–437.
Schwartz JI, Van Hecken A, De Schepper PJ, et al. Effect of MK-386, a novel inhibitor of Type 1 5α-reductase, alone and in combination with finasteride, on serum dihydrotestosterone concentrations in men. J Clin Endocrinol Metab 1996;81:2942–2947.
Bakshi RK, Rasmusson GH, Patel GF, et al. 4-Aza-3-oxo5α-androst-1-ene-17 β-N-aryl-carboxamides as dual inhibitors of human Type 1 and Type 2 steroid 5α-reductases. Dramatic effect of N-aryl substituents on Type 1 and Type 2 5α-reductase inhibitory potency. J Med Chem 1995;38:3189–3192.
Kojo H, Nakayama O, Hirosumi J, et al. Novel steroid 5α-reductase inhibitor FK 143: its dual inhibition against the two isozymes and its effect on transcription of the isozyme genes. Mol Pharmacol 1995;48:410–406.
Bramson HN, Hermann D, Batchelor KW, et al. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther 1997;282:1496–1502.
Hermann DJ, Davis IM, Wilson TH. Effects of G1198745 (GG745), a novel 5α-reductase (5AR) inhibitor, on dihydrotestosterone (DHT). Am Soc Clin Pharmacol Therapeut 1996;59:162.
Hobbs S, Hermann DJ, Gabriel T, et al. Marked suppression of dihydrotestosterone in men by a novel 5α-reductase inhibitor, G1198745. Fertil Steril 1998;70:S455.
Dreikorn K, Borkowski A, Braeckman J, et al. Other medical therapies, in 4th International Consultation on Benign Prostatic Hyperplasia (BPH). Denis L, Griffiths, K, Cockett ATK, et al. eds. Plymouth, UK:Plymbridge Distributors Ltd., 1998, pp.635–659.
Délos S, Iehlé C, Martin PM. Inhibition of the activity of “basic” 5α-reductase (Type 1) detected in DU 145 cells and expressed in insect cells. J Steroid Biochem Mol Biol 1994;48:347–352.
Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5α-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300–306.
Casarosa C, Coscio di Coscio M, Fratta M. Lack of effects of a lyposterolic extract of Seronoa repens on plasma levels of testosterone, follicle-stimulating hormone, and luteinizing hormone. Clin Ther 1988;5:585–588.
Strauch G, Perles P, Vergult G, et al. Comparison of finasteride (Proscar) and Serenoa repens (Permixon) in the inhibition of 5α-reductase in healthy male volunteers. Eur Urol 1994;26:247–252.
Braeckman J. The extract of Serenoa reopens in the treatment of benign prostatic hyperplasia: a multi-center open study. Clin Ther Res 1994;55:776–785.
Champault G, Patel JC, Bonnard A. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. Br J Clin Pharmacol 1984;18:461–462.
Rhodes L, Primka RL, Berman C, et al. Comparison of Finasteride (Proscar®), a 5α reductase inhibitor, and various commercial plant extracts in in vitro and in vivo 5α reductase inhibition. Prostate 1993;22:43–51.
Jonas A, Rosenblat G, Krapf D, et al. Cactus flower extracts may prove beneficial in benign prostatic hyperplasia due to inhibition of 5α-reductase activity, aromatase activity and lipid peroxidation. Urol Res 1998;26:265–270.
Tunn S, Krieg M. Alterations in the intraprostatic hormonal metabolism by the pollen extract Cernilton®, in Benign Prostatic Disease. Vahlensieck W, Rutishauser G, eds. Thieme Medical Publishers, New York, 1992:109–114.
Descotes JL, Rambeaud JJ, Deschaseaux P, Faure G. Placebo controlled evaluation of the efficacy and tolerability of Permixon® in benign prostatic hyperplasia after exclusion of placebo responders. Clin Drug Investig 1995;9:291–297.
Reece Smith H, Memon A, Smart CJ, Dewbury K. The value of Permixon in benign prostatic hypertrophy. Br J Urol 1986;58:36–40.
Carraro JC, Raynaud JP, Koch G, et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1098 patients. Prostate 1996;29:231–224.
Marks LS, Partin AW, Epstein JI, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol 2000;163:1451–1456.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2004 Springer Science+Business Media New York
About this chapter
Cite this chapter
Drisko, J.E., Yao, SL. (2004). Prostate Cancer Chemoprevention. In: Kelloff, G.J., Hawk, E.T., Sigman, C.C. (eds) Cancer Chemoprevention. Cancer Drug Discovery and Development. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-59259-767-3_13
Download citation
DOI: https://doi.org/10.1007/978-1-59259-767-3_13
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-61737-342-8
Online ISBN: 978-1-59259-767-3
eBook Packages: Springer Book Archive