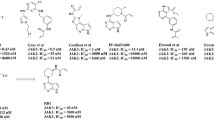
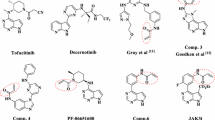
Abstract
During the past decade, covalent targeting has experienced a revival, especially in the kinase field. Addressing non-conserved cysteine residues by targeted covalent inhibitors has enabled the design of ligands with high selectivity in the kinome and has led to five currently approved drugs (1–5; early September 2018). Covalent inhibition was also the prime strategy for the selective targeting of JAK3, a member of the Janus kinase (JAK) family of non-receptor tyrosine kinases. JAKs are key regulators of the immune system. However, while the function of JAK3 is mainly limited to immune signaling, the remaining three JAK family members also fulfill other essential functions outside the immune system. Therefore, JAK3 has long been discussed as a promising target for the treatment of inflammatory and autoimmune disorders with limited side effects. Until recently, however, the development of sufficiently JAK3-selective small molecules was impeded by the high similarity of the JAKs’ ATP binding pockets. Addressing Cys909, which is a serine in the other JAK family members, with electrophilic warheads, has recently enabled the generation of JAK3 inhibitors with unprecedented selectivity in the JAK family and the kinome. These compounds have now paved the way for the in-depth examination of JAK3-dependent signaling in cells and in vivo. Current research efforts culminated in the development of PF-06651600, a phase II clinical candidate from Pfizer under investigation for the treatment of rheumatoid arthritis, inflammatory bowel disease, and alopecia areata.
In this chapter, the history of covalent JAK3 inhibitors will be reviewed followed by the detailed discussion of case studies on how covalent targeting of Cys909 enabled isoform- and kinome-wide selectivity for this promising therapeutic target.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ferguson FM, Gray NS (2018) Kinase inhibitors: the road ahead. Nat Rev Drug Discov 17:353–377. https://doi.org/10.1038/nrd.2018.21
Forster M, Gehringer M, Laufer SA (2017) Recent advances in JAK3 inhibition: isoform selectivity by covalent cysteine targeting. Bioorg Med Chem Lett 27:4229–4237. https://doi.org/10.1016/j.bmcl.2017.07.079
Pellegrini S, Dusanter-Fourt I (1997) The structure, regulation and function of the Janus kinases (JAKs) and the signal transducers and activators of transcription (STATs). Eur J Biochem 248:615–633. https://doi.org/10.1111/j.1432-1033.1997.00615.x
Clark JD, Flanagan ME, Telliez J-B (2014) Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 57:5023–5038. https://doi.org/10.1021/jm401490p
Ghoreschi K, Laurence A, O’Shea JJ (2009) Janus kinases in immune cell signaling. Immunol Rev 228:273–287. https://doi.org/10.1111/j.1600-065X.2008.00754.x
Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Et A (1994) Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266:1045–1047. https://doi.org/10.1126/science.7973659
Rochman Y, Spolski R, Leonard WJ (2009) New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol 9:480–490. https://doi.org/10.1038/nri2580
Gurniak CB, Berg LJ (1996) Murine JAK3 is preferentially expressed in hematopoietic tissues and lymphocyte precursor cells. Blood 87:3151–3160
Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T (1995) Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3:771–782
Roberts JL, Lengi A, Brown SM, Chen M, Zhou Y-J, O’Shea JJ, Buckley RH (2004) Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood 103:2009–2018. https://doi.org/10.1182/blood-2003-06-2104
Sohn SJ, Forbush KA, Nguyen N, Witthuhn B, Nosaka T, Ihle JN, Perlmutter RM (1998) Requirement for Jak3 in mature T cells: its role in regulation of T cell homeostasis. J Immunol 160:2130–2138
Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ (1993) Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73:147–157. https://doi.org/10.1016/0092-8674(93)90167-O
Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O’Shea JJ (2008) Therapeutic targeting of Janus kinases. Immunol Rev 223:132–142. https://doi.org/10.1111/j.1600-065X.2008.00644.x
Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Etages SGD, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si M-S, Paniagua R, Higgins J, Holm B, Reitz B, Zhou Y-J, Morris RE, O’Shea JJ, Borie DC (2003) Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302:875–878. https://doi.org/10.1126/science.1087061
Garber K (2013) Pfizer’s first-in-class JAK inhibitor pricey for rheumatoid arthritis market. Nat Biotech 31:3–4. https://doi.org/10.1038/nbt0113-3
Thoma G, Drückes P, Zerwes H-G (2014) Selective inhibitors of the Janus kinase Jak3 – are they effective? Bioorg Med Chem Lett 24:4617–4621. https://doi.org/10.1016/j.bmcl.2014.08.046
Press Announcements - FDA approves new treatment for moderately to severely active ulcerative colitis. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm609225.htm. Accessed 18 Oct 2018
Xeljanz. European Medicines Agency. https://www.ema.europa.eu/medicines/human/EPAR/xeljanz. Accessed 18 Oct 2018
Markham A (2017) Baricitinib: first global approval. Drugs 77:697–704. https://doi.org/10.1007/s40265-017-0723-3
Mullard A (2018) FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov 17:460
Mesa RA, Yasothan U, Kirkpatrick P (2012) Ruxolitinib. Nat Rev Drug Discov 11:103–104. https://doi.org/10.1038/nrd3652
Gonzales AJ, Bowman JW, Fici GJ, Zhang M, Mann DW, Mitton-Fry M (2014) Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther 37:317–324. https://doi.org/10.1111/jvp.12101
Thoma G, Nuninger F, Falchetto R, Hermes E, Tavares GA, Vangrevelinghe E, Zerwes H-G (2010) Identification of a potent Janus kinase 3 inhibitor with high selectivity within the Janus kinase family. J Med Chem 54:284–288. https://doi.org/10.1021/jm101157q
Lin TH, Hegen M, Quadros E, Nickerson-Nutter CL, Appell KC, Cole AG, Shao Y, Tam S, Ohlmeyer M, Wang B, Goodwin DG, Kimble EF, Quintero J, Gao M, Symanowicz P, Wrocklage C, Lussier J, Schelling SH, Hewet AG, Xuan D, Krykbaev R, Togias J, Xu X, Harrison R, Mansour T, Collins M, Clark JD, Webb ML, Seidl KJ (2010) Selective functional inhibition of JAK-3 is sufficient for efficacy in collagen-induced arthritis in mice. Arthritis Rheum 62:2283–2293. https://doi.org/10.1002/art.27536
Farmer LJ, Ledeboer MW, Hoock T, Arnost MJ, Bethiel RS, Bennani YL, Black JJ, Brummel CL, Chakilam A, Dorsch WA, Fan B, Cochran JE, Halas S, Harrington EM, Hogan JK, Howe D, Huang H, Jacobs DH, Laitinen LM, Liao S, Mahajan S, Marone V, Martinez-Botella G, McCarthy P, Messersmith D, Namchuk M, Oh L, Penney MS, Pierce AC, Raybuck SA, Rugg A, Salituro FG, Saxena K, Shannon D, Shlyakter D, Swenson L, Tian S-K, Town C, Wang J, Wang T, Wannamaker MW, Winquist RJ, Zuccola HJ (2015) Discovery of VX-509 (Decernotinib): a potent and selective Janus kinase 3 inhibitor for the treatment of autoimmune diseases. J Med Chem 58:7195–7216. https://doi.org/10.1021/acs.jmedchem.5b00301
Soth M, Hermann JC, Yee C, Alam M, Barnett JW, Berry P, Browner MF, Frank K, Frauchiger S, Harris S, He Y, Hekmat-Nejad M, Hendricks T, Henningsen R, Hilgenkamp R, Ho H, Hoffman A, Hsu P-Y, Hu D-Q, Itano A, Jaime-Figueroa S, Jahangir A, Jin S, Kuglstatter A, Kutach AK, Liao C, Lynch S, Menke J, Niu L, Patel V, Railkar A, Roy D, Shao A, Shaw D, Steiner S, Sun Y, Tan S-L, Wang S, Vu MD (2013) 3-Amido pyrrolopyrazine JAK kinase inhibitors: development of a JAK3 vs JAK1 selective inhibitor and evaluation in cellular and in vivo models. J Med Chem 56:345–356. https://doi.org/10.1021/jm301646k
Gehringer M, Pfaffenrot E, Bauer S, Laufer SA (2014) Design and synthesis of tricyclic JAK3 inhibitors with picomolar affinities as novel molecular probes. ChemMedChem 9:277–281. https://doi.org/10.1002/cmdc.201300520
Nakajima Y, Aoyama N, Takahashi F, Sasaki H, Hatanaka K, Moritomo A, Inami M, Ito M, Nakamura K, Nakamori F, Inoue T, Shirakami S (2016) Design, synthesis, and evaluation of 4,6-diaminonicotinamide derivatives as novel and potent immunomodulators targeting JAK3. Bioorg Med Chem 24:4711–4722. https://doi.org/10.1016/j.bmc.2016.08.007
Haan C, Rolvering C, Raulf F, Kapp M, Drückes P, Thoma G, Behrmann I, Zerwes H-G (2011) Jak1 has a dominant role over Jak3 in signal transduction through γc-containing cytokine receptors. Chem Biol 18:314–323. https://doi.org/10.1016/j.chembiol.2011.01.012
Thorarensen A, Banker ME, Fensome A, Telliez J-B, Juba B, Vincent F, Czerwinski RM, Casimiro-Garcia A (2014) ATP-mediated Kinome selectivity: the missing link in understanding the contribution of individual JAK kinase isoforms to cellular signaling. ACS Chem Biol 9:1552. https://doi.org/10.1021/cb5002125
Yoshida T, Kakizuka A, Imamura H (2016) BTeam, a novel BRET-based biosensor for the accurate quantification of ATP concentration within living cells. Sci Rep 6:39618. https://doi.org/10.1038/srep39618
Leonard WJ, Mitra S, Lin J-X (2016) Immunology: JAK3 inhibition—is it sufficient? Nat Chem Biol 12:308–310. https://doi.org/10.1038/nchembio.2066
Chaikuad A, Koch P, Laufer SA, Knapp S (2018) The cysteinome of protein kinases as a target in drug development. Angew Chem Int Ed 57:4372–4385. https://doi.org/10.1002/anie.201707875
Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM (1998) Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. PNAS 95:12022–12027. https://doi.org/10.1073/pnas.95.20.12022
Thorarensen A, Dowty ME, Banker ME, Juba B, Jussif J, Lin T, Vincent F, Czerwinski RM, Casimiro-Garcia A, Unwalla R, Trujillo JI, Liang S, Balbo P, Che Y, Gilbert AM, Brown MF, Hayward M, Montgomery J, Leung L, Yang X, Soucy S, Hegen M, Coe J, Langille J, Vajdos F, Chrencik J, Telliez J-B (2017) Design of a Janus Kinase 3 (JAK3) specific inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) allowing for the interrogation of JAK3 signaling in humans. J Med Chem 60:1971–1993. https://doi.org/10.1021/acs.jmedchem.6b01694
Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9:576–596
Brown GR, Bamford AM, Bowyer J, James DS, Rankine N, Tang E, Torr V, Culbert EJ (2000) Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg Med Chem Lett 10:575–579. https://doi.org/10.1016/S0960-894X(00)00051-2
Stepkowski SM, Kao J, Wang M-E, Tejpal N, Podder H, Furian L, Dimmock J, Jha A, Das U, Kahan BD, Kirken RA (2005) The Mannich base NC1153 promotes long-term allograft survival and spares the recipient from multiple toxicities. J Immunol 175:4236–4246. https://doi.org/10.4049/jimmunol.175.7.4236
Ross JA, Spadaro M, Rosado DC, Cavallo F, Kirken RA, Pericle F (2014) Inhibition of JAK3 with a novel, selective and orally active small molecule induces therapeutic response in T-cell malignancies. Leukemia 28:941–944. https://doi.org/10.1038/leu.2013.309
Styles M, Zeng J, Treutlein H, Wilks A, Kling M, Bu X, Burns C (2005) Selective kinase inhibitors
Goedken ER, Argiriadi MA, Banach DL, Fiamengo BA, Foley SE, Frank KE, George JS, Harris CM, Hobson AD, Ihle DC, Marcotte D, Merta PJ, Michalak ME, Murdock SE, Tomlinson MJ, Voss JW (2015) Tricyclic covalent inhibitors selectively target Jak3 through an active site thiol. J Biol Chem 290:4573–4589. https://doi.org/10.1074/jbc.M114.595181
Goldstein DM, Brameld KA, Verner E (2012) Azaindole derivatives as tyrosine kinase inhibitors
Goldstein D, Brameld K, Owens T (2014) Azaindole derivatives as JAK3 inhibitors
Ahearn S, Christopher M, Jung J, Pu Q, Rivkin A, Scott M, Witter D, Woo HC, Cash B, Dinsmore C, Guerin D (2013) Pyrrolopyrimidines as Janus kinase inhibitors
Smith GA, Uchida K, Weiss A, Taunton J (2016) Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling. Nat Chem Biol 12:373–379. https://doi.org/10.1038/nchembio.2056
London N, Miller RM, Krishnan S, Uchida K, Irwin JJ, Eidam O, Gibold L, Cimermančič P, Bonnet R, Shoichet BK, Taunton J (2014) Covalent docking of large libraries for the discovery of chemical probes. Nat Chem Biol 10:1066–1072. https://doi.org/10.1038/nchembio.1666
Gehringer M, Forster M, Laufer SA (2015) Solution-phase parallel synthesis of Ruxolitinib-derived Janus kinase inhibitors via copper-catalyzed Azide–Alkyne cycloaddition. ACS Comb Sci 17:5–10. https://doi.org/10.1021/co500122h
Forster M, Chaikuad A, Bauer SM, Holstein J, Robers MB, Corona CR, Gehringer M, Pfaffenrot E, Ghoreschi K, Knapp S, Laufer SA (2016) Selective JAK3 inhibitors with a covalent reversible binding mode targeting a new induced fit binding pocket. Cell Chem Biol 23:1335–1340. https://doi.org/10.1016/j.chembiol.2016.10.008
Tan L, Akahane K, McNally R, Reyskens KMSE, Ficarro SB, Liu S, Herter-Sprie GS, Koyama S, Pattison MJ, Labella K, Johannessen L, Akbay EA, Wong K-K, Frank DA, Marto JA, Look TA, Arthur JSC, Eck MJ, Gray NS (2015) Development of selective covalent Janus kinase 3 inhibitors. J Med Chem 58:6589–6606. https://doi.org/10.1021/acs.jmedchem.5b00710
Zhou W, Ercan D, Chen L, Yun C-H, Li D, Capelletti M, Cortot AB, Chirieac L, Iacob RE, Padera R, Engen JR, Wong K-K, Eck MJ, Gray NS, Jänne PA (2009) Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462:1070–1074. https://doi.org/10.1038/nature08622
Ge Y, Wang C, Song S, Huang J, Liu Z, Li Y, Meng Q, Zhang J, Yao J, Liu K, Ma X, Sun X (2018) Identification of highly potent BTK and JAK3 dual inhibitors with improved activity for the treatment of B-cell lymphoma. Eur J Med Chem 143:1847–1857. https://doi.org/10.1016/j.ejmech.2017.10.080
Elwood F, Witter DJ, Piesvaux J, Kraybill B, Bays N, Alpert C, Goldenblatt P, Qu Y, Ivanovska I, Lee H-H, Chiu C-S, Tang H, Scott ME, Deshmukh SV, Zielstorff M, Byford A, Chakravarthy K, Dorosh L, Rivkin A, Klappenbach J, Pan B-S, Kariv I, Dinsmore C, Slipetz D, Dandliker PJ (2017) Evaluation of JAK3 biology in autoimmune disease using a highly selective, irreversible JAK3 inhibitor. J Pharmacol Exp Ther 361:229–244. https://doi.org/10.1124/jpet.116.239723
Forster M, Chaikuad A, Dimitrov T, Döring E, Holstein J, Berger B-T, Gehringer M, Ghoreschi K, Müller S, Knapp S, Laufer SA (2018) Development, optimization, and structure–activity relationships of covalent-reversible JAK3 inhibitors based on a tricyclic Imidazo[5,4-d]pyrrolo[2,3-b]pyridine scaffold. J Med Chem 61:5350–5366. https://doi.org/10.1021/acs.jmedchem.8b00571
Kulagowski JJ, Blair W, Bull RJ, Chang C, Deshmukh G, Dyke HJ, Eigenbrot C, Ghilardi N, Gibbons P, Harrison TK, Hewitt PR, Liimatta M, Hurley CA, Johnson A, Johnson T, Kenny JR, Bir Kohli P, Maxey RJ, Mendonca R, Mortara K, Murray J, Narukulla R, Shia S, Steffek M, Ubhayakar S, Ultsch M, van Abbema A, Ward SI, Waszkowycz B, Zak M (2012) Identification of imidazo-pyrrolopyridines as novel and potent JAK1 inhibitors. J Med Chem 55:5901–5921. https://doi.org/10.1021/jm300438j
Chrencik JE, Patny A, Leung IK, Korniski B, Emmons TL, Hall T, Weinberg RA, Gormley JA, Williams JM, Day JE, Hirsch JL, Kiefer JR, Leone JW, Fischer HD, Sommers CD, Huang H-C, Jacobsen EJ, Tenbrink RE, Tomasselli AG, Benson TE (2010) Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6. J Mol Biol 400:413–433. https://doi.org/10.1016/j.jmb.2010.05.020
Robers MB, Dart ML, Woodroofe CC, Zimprich CA, Kirkland TA, Machleidt T, Kupcho KR, Levin S, Hartnett JR, Zimmerman K, Niles AL, Ohana RF, Daniels DL, Slater M, Wood MG, Cong M, Cheng Y-Q, Wood KV (2015) Target engagement and drug residence time can be observed in living cells with BRET. Nat Commun 6:1–10. https://doi.org/10.1038/ncomms10091
Telliez J-B, Dowty ME, Wang L, Jussif J, Lin T, Li L, Moy E, Balbo P, Li W, Zhao Y, Crouse K, Dickinson C, Symanowicz P, Hegen M, Banker ME, Vincent F, Unwalla R, Liang S, Gilbert AM, Brown MF, Hayward M, Montgomery J, Yang X, Bauman J, Trujillo JI, Casimiro-Garcia A, Vajdos FF, Leung L, Geoghegan KF, Quazi A, Xuan D, Jones L, Hett E, Wright K, Clark JD, Thorarensen A (2016) Discovery of a JAK3-selective inhibitor: functional differentiation of JAK3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem Biol 11:3442–3451. https://doi.org/10.1021/acschembio.6b00677
He L, Pei H, Lan T, Tang M, Zhang C, Chen L (2017) Design and synthesis of a highly selective JAK3 inhibitor for the treatment of rheumatoid arthritis. Arch der Pharm 350:1700194. https://doi.org/10.1002/ardp.201700194
Pei H, He L, Shao M, Yang Z, Ran Y, Li D, Zhou Y, Tang M, Wang T, Gong Y, Chen X, Yang S, Xiang M, Chen L (2018) Discovery of a highly selective JAK3 inhibitor for the treatment of rheumatoid arthritis. Sci Rep 8:5273. https://doi.org/10.1038/s41598-018-23569-y
He L, Shao M, Wang T, Lan T, Zhang C, Chen L (2018) Design, synthesis, and SAR study of highly potent, selective, irreversible covalent JAK3 inhibitors. Mol Divers 22:1–16. https://doi.org/10.1007/s11030-017-9803-2
Jackson PA, Widen JC, Harki DA, Brummond KM (2017) Covalent modifiers: a chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J Med Chem 60:839–885. https://doi.org/10.1021/acs.jmedchem.6b00788
Flanagan ME, Abramite JA, Anderson DP, Aulabaugh A, Dahal UP, Gilbert AM, Li C, Montgomery J, Oppenheimer SR, Ryder T, Schuff BP, Uccello DP, Walker GS, Wu Y, Brown MF, Chen JM, Hayward MM, Noe MC, Obach RS, Philippe L, Shanmugasundaram V, Shapiro MJ, Starr J, Stroh J, Che Y (2014) Chemical and computational methods for the characterization of covalent reactive groups for the prospective design of irreversible inhibitors. J Med Chem 57:10072–10079. https://doi.org/10.1021/jm501412a
Allimuthu D, Adams DJ (2017) 2-Chloropropionamide as a low-reactivity electrophile for irreversible small-molecule probe identification. ACS Chem Biol 12:2124–2131. https://doi.org/10.1021/acschembio.7b00424
Gehringer M, Forster M, Pfaffenrot E, Bauer SM, Laufer SA (2014) Novel hinge-binding motifs for Janus kinase 3 inhibitors: a comprehensive structure–activity relationship study on Tofacitinib Bioisosteres. ChemMedChem 9:2516–2527. https://doi.org/10.1002/cmdc.201402252
Kempson J, Ovalle D, Guo J, Wrobleski ST, Lin S, Spergel SH, Duan JJ-W, Jiang B, Lu Z, Das J, Yang BV, Hynes J, Wu H, Tokarski J, Sack JS, Khan J, Schieven G, Blatt Y, Chaudhry C, Salter-Cid LM, Fura A, Barrish JC, Carter PH, Pitts WJ (2017) Discovery of highly potent, selective, covalent inhibitors of JAK3. Bioorg Med Chem Lett 27:4622–4625. https://doi.org/10.1016/j.bmcl.2017.09.023
Acknowledgments
The authors thank Kristine Schmidt for proofreading. M.G. acknowledges financial support from the Institutional Strategy of the University of Tübingen (ZUK 63, German Research Foundation) and the Postdoctoral Fellowship Program of the Baden-Württemberg Stiftung.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest: The authors declare that they have no conflict of interest.
Funding: While preparing the manuscript, M.G. received funding from the Institutional Strategy of the University of Tübingen (ZUK 63, German Research Foundation) and the Postdoctoral Fellowship Program of the Baden-Württemberg Stiftung.
Ethical Approval: This chapter does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gehringer, M., Forster, M. (2020). Covalent Janus Kinase 3 Inhibitors. In: Laufer, S. (eds) Proteinkinase Inhibitors. Topics in Medicinal Chemistry, vol 36. Springer, Cham. https://doi.org/10.1007/7355_2020_96
Download citation
DOI: https://doi.org/10.1007/7355_2020_96
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68179-1
Online ISBN: 978-3-030-68180-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)