Abstract
The radiocesium contamination in mushrooms and in mushroom logs is a matter of concern for the forestry industry. To know the contamination situation precisely and the future for mushroom log production, the 137Cs distribution in six fields cultivating oak trees in Tamura city, Fukushima, was investigated in 2015. The 137Cs concentration in new branches was found to correlate with that in the wood. This result suggests that the 137Cs concentration in tree trunks could be estimated without felling based on the 137Cs concentration in new branches. In addition, trees grown in one of the six fields was found to have very low 137Cs concentration even though the concentration of 137Cs in the soil was high. The impact of the nutritional conditions on the 137Cs absorption in the oak seedlings grown hydroponically was also investigated by a radiotracer experiment.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
In Japanese cuisine, shiitake mushroom (Lentinula edodes) is one of the essential and traditional ingredients that can be grilled, boiled, etc. Dried shiitake is also an important ingredient as it produces a characteristic liquid full of umami when it is reconstituted in water.
Although shiitake grows naturally in the wild in all corners of Japan, almost all shiitake in the marketplace is grown commercially. Cultivation of shiitake in Japan is considered to have begun in the 1600s. There are two methods for cultivating shiitake: using either logs or sawdust blocks as the cultivation bed. Shiitake produced using logs is reported to have a better flavor, although the productivity is not so high.
The annual production of shiitake in Japan in 2010 was 77,000 t, and the production value was 76 billion yen, which was the highest among all kinds of mushrooms. The production of shiitake has been rather even between regions in Japan. Fukushima prefecture was the seventh largest producer of shiitake; 3500 t was produced in 2010, which accounts for 5% of total Japanese shiitake production. However, as of 2014, the annual production in Fukushima prefecture decreased by approximately 50% due to the accident in the Fukushima Daiichi Nuclear Power Plant, whereas on a national level the annual production decreased only slightly. Shiitake mushroom production using logs has been particularly damaged by the accident as these mushrooms are normally cultivated outdoor.
Besides mushroom production, Fukushima prefecture has been a major producer of mushroom logs. In Japan, several hardwood species, specifically oaks, including Quercus serrata “Konara”, Quercus crispula “Mizunara”, and Quercus acutissima “Kunugi”, are grown for approximately 20 years and used for mushroom logs. Meanwhile, among the 47 prefectures, only nine are self-sufficient in mushroom logs. The prefectures not self-sufficient in logs need to import logs from prefectures that have a surplus, and up until 2011, Fukushima was the prefecture that supplied the most logs to other prefectures. Radiocesium concentration in mushroom logs must be less than 50 Bq/kg in order not to produce shiitake containing more than 100 Bq/kg of radiocesium, which is the Japanese standard limit for general foods. To date, it is actually very difficult to produce mushroom logs containing radiocesium less than 50 Bq/kg in the forest contaminated directly by radioactive fallout. Consequently, oak tree forests in large areas of Fukushima prefecture have not been harvested since the accident, and mushroom growers in many parts of Japan have experienced a shortage of mushroom logs.
11.2 Objective and Research Field
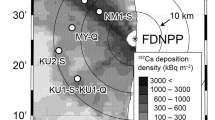
“When can mushroom-log-production resume?” This question is the one that scientists and investigators are frequently asked by forestry workers in Fukushima. Considering that the cultivation period from planting to harvesting spans 20 years, and another 10 years until the shoots from the stump mature, the prospect of restarting log production is essential for forestry workers to know if they have a future in this industry. In this context, the purpose of this research was to assess the prospect of resuming mushroom-log production in contaminated areas of Fukushima. Forest contamination was investigated in three areas (A, B, C) in Tamura city in collaboration with the Fukushima Chuo Forest Cooperative (Miura 2016), located about 20 km from the Fukushima Daiichi nuclear power plant (Fig. 11.1). Air dose rate at a height of 1 m above the ground in the areas A, B, C, was 0.43 ~ 0.5, 0.3 ~ 0.35, and 0.2 ~ 0.25 μSv/h, respectively, in the autumn of 2015. In area A, three sites were provided as the research field (i.e., A-1, A-2, and A-3), and in area C, 2 sites (i.e., C-1 and C-2) were provided. Therefore, six sites in total were established in the three areas (Table 11.1).
11.3 Field Investigation
11.3.1 Sample Collection
Details of the trees sampled in the six sites are shown in Table 11.1. Konara, Mizunara, and Kunugi trees are deciduous trees, and thus they had no leaves in March 2011 when the accident occurred. Planted trees contaminated by direct deposition of radionuclides were investigated in area A. On the other hand, old trees having direct contamination on their surface had been coppiced in area B and C, and the coppiced trees developed from the stump were targeted (Table 11.1). From May to September 2015, leaves on branches were sampled monthly in sites A-1, A-2, B, and C-2. In November 2015, planted trees in area A were cut down, and the trunks, the leaves, and the new branches which had grown in the current growing season were sampled (Fig. 11.2). The trunk was further separated into bark and wood (Fig. 11.2). Leaves, new branches, and trunks were also sampled in areas B and C in November 2015 (Fig. 11.2). For soil analysis, samples were collected at a depth of 0–5 cm (surface soil) and 5–15 cm (Fig. 11.2). The number of replicates varied between 14 and 21 depending on the sample type. Radioactivity of the samples was measured using either Ge semiconductor detector (Kobayashi et al. 2016) or NaI scintillation counter (Endo et al. 2015).
11.3.2 Property of the Soil
The bulk density of the soils in site A-1 and A-2 were almost twice as high as the soil in other sites (Table 11.2). This could be because the ground of these two sites was artificially developed, while others have natural ground covered with a thick A1 layer.
The content of exchangeable ions was determined by the ammonium acetate extraction method. The content of exchangeable potassium, magnesium, and especially calcium in A-1 soil was surprisingly high (Table 11.2). In addition, soil pH in site A-1 was greater than 6 (Table 11.2). The reason for the characteristic soil property in site A-1 should be found in the use history of this field. In fact, site A-1 had been a corn farm before oak trees were planted. For better corn production, this field was limed intensively in the past.
11.3.3 137Cs Concentrations in Above Ground Parts
Considering the relatively small variation in soil 137Cs concentration among the six sites, the difference in the 137Cs concentration in oak trees was unexpectedly large (Fig. 11.3). Particularly, in site A-1, it was surprising that the 137Cs concentration was mostly lower than 25 Bq/kg in new branches and leaves, and even lower in the wood (Fig. 11.3). On the other hand, the 137Cs concentrations in the new branches and leaves in site A-2 was mostly between 150 Bq/kg and 350 Bq/kg (Fig. 11.3). This means that the radiocesium contamination in trees on site A-1 was one-tenth of those on site A-2, although the soil 137Cs concentration was not different between sites. Contrary, the 137Cs concentration level in the soil in site A-3 was nearly 2.5-fold higher than site A-2, and then the 137Cs concentration level in oak trees in site A-3 was also 2.5-fold higher than that in site A-2 (Fig. 11.3). Characteristically, the ratio between branch 137Cs concentration and soil 137Cs concentration was 0.13 for site A-2 and 0.17 for site A-3, while the ratio was 0.008 for site A-1. A similar relationship between soil and coppiced tree contamination in sites C-1 and C-2 was found. The 137Cs concentration in trees on site C-2 was 2-fold higher than on site C-1, though the soils in these sites were contaminated similarly (Fig. 11.3). In total, current 137Cs concentration level in oak trees cannot be simply determined by the amount of 137Cs deposition in the forest (Table 11.2). Therefore, we aimed to understand the factors that potentially have an affect on 137Cs concentration in oak trees, as described in the following Sect. 11.5.
137Cs concentration in surface soil (a), new branches grown in a current growing season (b), leaf (c), wood (d), bark (e), and trunk composed of wood and unsuberized young bark (f). The hinges refer to the 25th percentile (right gray) and 75th percentile (dark gray), and the whisker lines indicate maximum and minimum values
In area A, 137Cs concentration in each tree part in site A-1 showed low 137Cs concentration, whereas all parts of trees in site A-3 showed high 137Cs concentration (Fig. 11.3). This indicates the possibility that the 137Cs concentration in wood and bark, which are the parts used as the mushroom log, can be estimated nondestructively based on the 137Cs concentration in the new branches or leaves. Figure 11.4 shows the correlation in 137Cs concentration in the different parts of the trees. There was a high correlation (R2 = 0.8564) between the new branches and wood (Fig. 11.4). The 137Cs concentration in wood was approximately one-third of that in the new branches, regardless of the tree age (Fig. 11.4). Similarly, the 137Cs concentration in leaves and wood was positively correlated (R2 = 0.8583) (Fig. 11.4). On the other hand, the correlation between the new branches and bark was lower (Fig. 11.4). Given that the bark provides the pathway for radiocesium contamination into the internal tissues of trees after direct deposition on the surface, the original contamination level in the bark can be assumed to have been correlated with the contamination level in other tree parts. However, over the last 5 years (from 2011 to 2015), surface contamination may have been washed out by rain, or the contaminated bark fell away as the tree grew. Consequently, bark contamination level in each individual tree could have changed unevenly, and thus reducing the correlation between bark 137Cs concentration and the others tree parts. Therefore, 137Cs concentration in leaves and new branches is a potential indicator to estimate the 137Cs concentration in wood, but leaves and new branches cannot be used to estimate the contamination level in the trunk when radioactive compounds were directly deposited on the bark.
The coppiced trees in areas B and C had young trunks without suberized mature bark, which was not contaminated directly by fallout (Table 11.1). In these trunks, 137Cs concentration was positively and highly correlated with 137Cs concentration in new branches (Fig. 11.4). The slope of the line was 0.84 (Fig. 11.4), suggesting that 137Cs concentration is higher in the younger branch.
11.3.4 Seasonal Variation in Leaf 137Cs Concentration
Knowledge about the accumulation and transport of cesium inside a tree body can be essential to think about the long-term changes in radiocesium contamination in trees. In the current study, the seasonal variation of 137Cs concentration in leaves was investigated. Oak trees in Tamura city come into leaf in May, and the senescent leaves are mostly shed by November (Fig. 11.5). We investigated 42 kunugi trees in area A, 21 mizunara trees in site B, and 21 konara trees in site C-2. No common pattern in the change of 137Cs concentration in leaves through the green period was found. Roughly classified, there were 3 patterns: (1) peaking in summer, (2) continuously decreasing, and (3) no variation. The first pattern predominated in site B, but otherwise, the three patterns were found randomly in each site (Fig. 11.5).
Seasonal variation in mineral concentration in leaves can be determined by root uptake and internal translocation from reserves in various living tissues. In peach trees, reserves have been estimated to supply approximately 40% of the requirement in phosphorous and potassium for new shoot growth during the first 8 weeks after bud-break (Stassen et al. 1983). The quantity of several minerals absorbed via roots was estimated in mature pistachio trees, and potassium uptake was found to occur mostly after spring flush season (Rosecrance et al. 1996). On the other hand, in case of oak trees with 137Cs contamination primarily on their shoot surface and then via root uptake, the ratio between the quantity of 137Cs taken up by roots and the volume of 137Cs reserved within the tissues can be different for each individual tree and also can vary year by year. This situation could cause the variations in the pattern of seasonal change in leaf 137Cs concentration.
11.4 Comparison Between 137Cs Distribution and 133Cs Distribution in Wood, Bark, and New Branches
The clear correlation observed between 137Cs concentration in new branches and wood (Fig. 11.4) indicated that some physiological system determined 137Cs distribution. We propose that it is the same system that regulates 133Cs uptake, accumulation and translocation in oak trees. Thus, the 133Cs distributions in wood, bark, and new branches in trees on site A-2 and A-3 were comparatively analyzed using ICP-MS after the radioactivity in the samples was determined. The result showed that 133Cs concentration in wood positively correlated with that in new branches (R2, 0.68; slope, 0.22) (Fig. 11.6). Accordingly, the 3: 1 ratio between 137Cs concentration in branches and wood (Fig. 11.4), is assumed to increase over the years to gradually approach a ratio of 5:1 (Fig. 11.6). Nevertheless, we can make an approximate estimation of the 137Cs concentration in wood based on the 137Cs concentrations in new branches. In contrast to wood, the concentration of 133Cs in bark only weakly correlated with 133Cs in new branches (R2, 0.4836) (Fig. 11.6). Nevertheless, given that the difference in concentration between bark and wood was very small in 133Cs compared to 137Cs (Figs. 11.4 and 11.6), and because wood comprises the largest proportion of the trunk volume, it could be possible that 137Cs concentration in new branches can be used as an indicator of 137Cs concentration in the trunk after the highly contaminated bark tissue falls off and the 137Cs distribution becomes similar to that of 133Cs.
11.5 Extra Field Investigation to Evaluate the Impact of Field Use History on the Current 137Cs Content in Trees
Because of the unexpectedly low contamination level of kunugi in site A-1 (Fig. 11.3), we tried to identify a factor that could be potentially affecting 137Cs concentration in these trees. The distinctive feature of site A-1 is that it used to be a field that grew corn. Additionally, the trees in site A-1 were the youngest among trees in site A-2 and A-3, indicating the dilution effect due to the higher growth rate needs to be also considered. Therefore, we investigated 9-year-old planted trees in another three sites in Tamura city in December 2015. Site D used to be a farm before the oak trees were planted. Site E and site F are artificial forests covered with bamboo grass (Fig. 11.7). Soil pH was relatively high in site D (Table 11.3), though it was not as high as in site A-1 (Table 11.2). The concentration of 137Cs in surface soil and new branches was measured. In the new branches of both konara and kunugi on site D, the 137Cs concentration was found to be lower than the detection limit (<56 Bq/kg), resulting in the ratio between branch 137Cs concentration and soil 137Cs concentration of less than 0.01 (Table 11.3). On the other hand, the branch-to-soil ratios in sites E and F were higher than 0.04 (Table 11.3). In conclusion, the site condition that is being influenced by the field use history appears to be affecting the 137Cs concentration in trees of the same age. Further studies will be required to elucidate what environmental variable in the site is controlling the 137Cs concentration in trees, and what is the mechanism.
11.6 137Cs Tracer Experiment Using Hydroponically Grown Young Oak Seedlings
In crop plants, uptake and translocation of cesium are known to be altered depending on the cultivation medium (Nobori et al. 2014). Therefore, the effect of nutritional conditions on cesium transport in oak trees was analyzed by the 137Cs tracer experiment (Kobayashi et al. 2016). Konara trees were hydroponically cultured at 25 °C in the plant growth chamber. Two different types of nutrient solution were used; half-strength Hoagland’s solution and Kimura-B. The concentration of minerals in Hoagland’s solution is generally higher than in Kimura-B. On the day of the experiment, the trees supplied with rich nutrition by Hoagland’s solution developed more leaves and bigger roots compared to the trees with poor nutrition (Fig. 11.8). Nevertheless, 137Cs uptake by roots for 3 h was noticeably smaller in the trees with rich nutrition (Fig. 11.8). We demonstrated that the mechanisms for mediating Cs uptake would appear to be down-regulated in response to the rich nutrition in rhizosphere.
The behavior of 137Cs deposited on the surface of a young stem was visually analyzed to consider the radiocesium movement after reaching the vasculature tissues inside the trunk. The 137Cs radiograph showed the rapid translocation of radiocesium toward the leaf and roots within 5 days (Fig. 11.9). Interestingly, not all the root tissue accumulated 137Cs (Fig. 11.9), and furthermore, a little portion of 137Cs was released to the nutrient solution during the next 5 days.
11.7 Conclusion
The contamination level in oak trees is practically in proportion to the contamination level in the soil in which the tree is growing, which can be a mirror of the amount of radiocesium deposition after the nuclear power plant accident. However, there can be additional environmental factors modulating the radiocesium concentration in trees 4 years after the accident. One factor could be a chemical property of the soil, including pH and nutrient content. Further investigation to identify the factors can allow the selection of low contaminated trees even today, and take measures to gradually reduce the radiocesium concentration in the trunk of the hardwood trees grown in the contaminated forest.
References
Endo I, Ohte N, Iseda K, Tanoi K, Hirose A, Kobayashi NI, Murakami M, Tokuchi N, Ohashi M (2015) Estimation of radioactive 137-cesium transportation by litterfall, stemflow and throughfall in the forests of Fukushima. J Environ Radioact 149:176–185
Kobayashi NI, Sugita R, Nobori T, Tanoi K, Nakanishi TM (2016) Tracer experiment using 42K+ and 137Cs+ revealed the different transport rates of potassium and caesium within rice roots. Funct Plant Biol 43:151–160
Miura S (2016) The effects of radioactive contamination on the forestry industry and commercial mushroom-log production in Fukushima, Japan. In: Agricultural implications of the Fukushima nuclear accident. The first three years. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55828-6_12
Nobori T, Kobayashi NI, Tanoi K, Nakanishi TM (2014) Effects of potassium in reducing the radiocesium translocation to grain in rice. Soil Sci Plant Nutr 60:772–781
Rosecrance RC, Weinbaum SA, Brown PH (1996) Assessment of nitrogen, phosphorus, and potassium uptake capacity and root growth in mature alternate-bearing pistachio (Pistacia vera) trees. Tree Physiol 16:949–956
Stassen PJC, Du Preez M, Stadler JD (1983) Reserves in full-bearing peach trees. Macro-element reserves and their role in peach trees. DFGA 33:200–206
Acknowledgement
This project was carried out with cooperation from the following members; Dr. Satoru Miura (Forest Research and Management Organization), Dr. Nobuhito Sekiya (Mie University), Dr. Daisuke Takata (Fukushima University), Dr. Stephan Bengtsson (Formerly at Fukushima University), Dr. Kazuhisa Yamasaki (The University of Tokyo), Dr. Naoto Nihei (The University of Tokyo), Dr. Keitaro Tanoi (The University of Tokyo), and Mr. Ryosuke Ito (Former master-course student). This project was supported in part by The Specific Research Grant 2015 for East Japan Great Earthquake Revival by The New Technology Development Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Kobayashi, N.I., Ito, R., Masumori, M. (2019). Radiocesium Contamination in Forests and the Current Situation of Growing Oak Trees for Mushroom Logs. In: Nakanishi, T., O`Brien, M., Tanoi, K. (eds) Agricultural Implications of the Fukushima Nuclear Accident (III). Springer, Singapore. https://doi.org/10.1007/978-981-13-3218-0_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-3218-0_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3217-3
Online ISBN: 978-981-13-3218-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)