Abstract
Invasive coronary physiology is a key instrument in decision-making for the interventional cardiologist. Fractional flow reserve has been well validated in chronic stable coronary artery disease. Its practical applications have expanded into other clinical situations such as acute coronary syndrome (ACS) including ST-elevation myocardial infarction (STEMI). Recently, other invasive indices of coronary physiology including instantaneous wave-free ratio (iFR), index of microvascular resistance (IMR), hyperemic stenosis resistance (HSR), and coronary flow reserve (CFR) have been explored in the context of ACS. This review will focus on the fundamentals and role of physiologic lesion assessment in the ACS patient.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Coronary artery disease
- Coronary flow reserve
- Coronary hemodynamics
- Fractional flow reserve
- iFR
- Angiography
- STEMI
- Acute coronary syndrome
15.1 Introduction
Although coronary artery disease (CAD), the most common cause of morbidity and mortality in the USA, is identified principally by coronary angiography, the relationship between the degrees of angiographic narrowing (% stenosis) is poorly correlated to its functional response (e.g., stress testing or intracoronary physiology). Over the past two decades, the use of in-lab coronary physiology has demonstrated that angiography alone is not accurate in determining ischemia for intermediate lesions. Compared with angiographically guided percutaneous coronary intervention (PCI), physiology-guided PCI is associated with improved clinical outcomes and cost-effectiveness. The most commonly used invasive physiologic indices include fractional flow reserve (FFR), coronary flow reserve (CFR), and instantaneous wave-free ratio (iFR). Understanding applied coronary physiology and the tools for measuring it in the cath lab is paramount to best clinical decision-making in interventional cardiology.
While FFR has been well validated in patients with chronic stable coronary artery disease, in ACS, physiological assessment of the culprit coronary artery is not performed because reduced flow to the myocardial bed can lead to false negatives. However, FFR can be measured in the non-culprit vessels because theoretically it is presumed that flow is not reduced to myocardial regions remote for the culprit infarct vessel territory. This chapter will be to review the different methods of invasive physiological assessment of coronary stenoses and their outcomes with a focus on their practical applications in patients with ST-elevation myocardial infarction (STEMI) and other ACS.
15.2 ACS and FFR Case
A 58-year-old man presented with 1 day of intermittent chest pain. His medical history was significant for hypertension and hyperlipidemia. The ECG showed anterior T wave inversions and his troponin was 4. Urgent cardiac catheterization revealed a 95% stenosis in the proximal left circumflex (LCX) artery and a 60% stenosis in the proximal left anterior descending (LAD) artery (Figs. 15.1 and 15.2). The presumed culprit vessel, the left circumflex artery, was stented (Fig. 15.3). Turning to the LAD, should we proceed to stent, defer and treat medically, or measure FFR and treat based on the ischemic potential of the lesion? Based on the evidence (see below), FFR of the non-infarct LAD artery is not only reasonable but favored to reduce operator uncertainty and shorten the current and future hospitalizations. As the lesion was fairly remote from the injured lateral wall myocardial zone, FFR should be valid (especially if positive). FFR of the proximal LAD lesion was 0.78 (Fig. 15.4) and stented (Fig. 15.5) in the same procedure. The patient was discharged uneventfully.
15.3 Fractional Flow Reserve Foundations
FFR is the ratio of the mean coronary pressure distal (Pd) to a stenosis to the mean aortic pressure (Pa) at maximal hyperemia when myocardial resistance is at its presumed absolute minimum. This condition permits pressure and flow to be linearly related, and thus the ratio represents the fraction of normal coronary blood flow across a stenosis with a normal value being 1 (i.e., Pd = Pa). FFR requires the induction of maximal hyperemia, most commonly with an intravenous infusion or intracoronary bolus of adenosine. Initial validation studies in stable angina patients have shown that an FFR <0.75, a reduction in coronary perfusion pressure by 25% of normal, was indicative of ischemia on stress testing. Additional investigations comparing FFR to different types of stress tests found that nearly all were negative when FFR > 0.80 and positive with FFR < 0.75, thereby establishing a gray zone of uncertainty. Clinical trials opted to use the upper end of the gray zone, 0.80 as the clinical threshold for outcome decision-making. This threshold is endorsed by the American College of Cardiology (ACC), American Heart Association (AHA), Society for Cardiovascular Angiography and Interventions (SCAI), and European Society of Cardiology (ESC) in their guidelines for PCI.
The outcomes of patients within the gray zone of 0.75–0.80 have been studied by Adjedj J et al. [1] who found that among 238 patients with gray-zone FFR, revascularization was associated with a significantly reduced risk of major adverse cardiovascular events (MACE) compared with medical therapy alone (Fig. 15.6) [1, 2]. On the other hand, the risk of MACE was not significantly different between deferred and revascularized lesions for FFR ≥0.76 (including the gray zone) in the large, prospective, multicenter Interventional Cardiology Research Incooperation Society Fractional Flow Reserve (IRIS-FFR) registry [3]. For patients with an FFR between 0.75 and 0.8, the decision to revascularize requires the operators’ synthesis of findings based on the clinical context of the patient.
MACE-free survival (%) in patients of the medical therapy group stratified by FFR strata (log-rank, 15; P < 0.001). FFR fractional flow reserve, MACE major adverse cardiovascular event. Source: Adjedj J, et al. Circulation. 2016;133:502–508 [1]
15.4 Fundamentals of Fractional Flow Reserve in STEMI and Other ACS
The utility of FFR to guide revascularization of intermediate lesions in patients with chronic stable multivessel coronary artery disease has been validated in several landmark clinical trials including the Fractional Flow Reserve to Determine the Appropriateness of Angioplasty in Moderate Coronary Stenosis (DEFER), FFR Versus Angiography for Multivessel Evaluation (FAME 1), and FFR-Guided Percutaneous Coronary Intervention Plus Medical Treatment Versus Medical Treatment Alone in Patients with Stable Coronary Artery Disease (FAME 2) trials [4,5,6]. The utility of FFR in acute coronary syndrome (ACS) has been controversial.
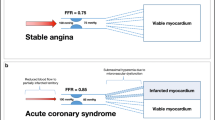
During a STEMI, the degree of damage to the infarct-related or culprit myocardial bed in the first hours of the injury makes the accuracy of FFR in the culprit artery highly questionable when >0.80. Since microcirculatory injury reduces flow, a low flow across a stenosis can produce a high FFR. Depending on the severity of the infarct, flow may recover in the days following the infarct, and hence the prior negative FFR may become positive, i.e., <0.80. To recap, a sub-hyperemic response to adenosine would result in an underestimation of stenosis severity so that an FFR >0.80 at the time of STEMI may initially be considered nonsignificant, but it may decrease several days later as the injured myocardial bed recovers and flow increases to the area. In other words, it may create a false negative. However, an FFR of <0.80 is truly functionally significant (Fig. 15.7).
STEMI and FFR cartoon on influence of myocardial bed size. The upper image shows a 50% stenosis supplying a large myocardial bed. Because flow to the bed is large, the FFR is 0.75. After a myocardial infarction of this bed, the injured myocardium has lower flow, and despite a more severe stenosis of 85%, the FFR is now higher 0.83
In the case of the non-culprit ACS artery, the flow to the myocardium supplied by non-infarct-related arteries should not be as affected as the culprit artery. The microcirculation should be normal, and FFR should accurately reflect the functional significance of a lesion in a non-culprit artery. All studies investigating the utility of FFR in STEMI or other ACS have assessed the non-culprit arteries. There are several studies that have found the microcirculation of the remote region can be abnormal [7], and the closer the non-culprit vessel is to the STEMI territory, the higher the potential for a false negative (due to impaired border zone microcirculatory function and less inducible hyperemia).
15.5 Fractional Flow Reserve and STEMI Revascularization Guidance
Traditionally, treating only the culprit vessel during STEMI has been considered superior to treating all lesions supported in part by several meta-analyses, and non-randomized registry studies showed that treating all vessels at the same time as the STEMI culprit was associated with more adverse events. Even the 2015 update of the 2013 ACC/AHA/SCAI STEMI guidelines gave a class III (do not do) recommendation to intervening on a non-infarct-related artery at the time of primary PCI in patients who are hemodynamically stable [8].
However, several recent studies have shown FFR is accurate in ACS, including the Fractional Flow Reserve Versus Angiography in Guiding Management to Optimize Outcomes in Non-ST-Elevation Myocardial Infarction Cardiac Magnetic Resonance (FAMOUS NSTEMI CMR) substudy that showed an excellent accuracy of FFR <0.80 for predicting perfusion defects on cardiac magnetic resonance [9]. Furthermore, multiple trials including the Preventive Angioplasty in Acute Myocardial Infarction (PRAMI), Complete Versus Lesion-Only Primary PCI Trial (CvLPRIT), and Third Danish Study of Optimal Acute Treatment of Patients With STEMI: Primary PCI in Multivessel Disease (DANAMI3-PRIMULTI) have shown that revascularizing non-culprit arteries in a STEMI, either at the time of primary PCI or later in a staged manner, reduce risk of MACE by a relative 44–65% compared with culprit-only PCI (Fig. 15.8) [10,11,12].
The PRAMI trial looked at 435 patients with acute STEMI and multivessel coronary artery disease. Patients were randomized to receive immediate preventive PCI in non-infarct arteries with stenosis >50% or no further PCI procedures. Recruitment was stopped early due to a highly significant between-group difference in the incidence of primary outcome favoring preventive PCI. Preventive PCI reduced the combined rate of cardiac death, nonfatal MI, or refractory angina by 65%, an absolute risk reduction of 14% over 23 months. Of note, revascularization was not included as a primary outcome. In this study, the severity of disease in non-infarct arteries was determined by angiographic assessment alone [10]. The effect of using FFR to determine the significance of disease in non-infarct arteries in STEMI patients was subsequently studied in DANAMI3-PRIMULTI and COMPARE-ACUTE trials.
DANAMI3-PRIMULTI was an open-label, randomized controlled trial that enrolled 627 patients with STEMI who had ≥1 clinically significant coronary stenosis in addition to the culprit lesion. After successful PCI to the culprit lesion, patients were randomized into no further invasive treatment or complete FFR-guided revascularization before discharge. A threshold of FFR <0.80 was used, and FFR was performed 2 days after primary PCI to avoid the risk of invalid FFR measurements inferred from acute changes in macrovascular tone or microvascular flow obstruction. The primary endpoint of a composite of all-cause mortality, nonfatal reinfarction, and ischemia-driven revascularization of lesions in non-infarct-related arteries was significantly lower in the complete revascularization group (13%) compared with the infarct-related-only group (22%). The favorable effect was driven by significantly fewer revascularizations. A substudy of the DANAMI3-PRIMULTI trial found that the benefit of staged FFR-guided complete revascularization was observed primarily in patients with three-vessel disease and at least one non-infarct-related stenosis with a ≥ 90% diameter (Fig. 15.9) [13].
Results from the DANAMI3-PRIMULTI study. In STEMI patients, the primary composite endpoint of all cause death, reinfarction, and ischemia-driven revascularization was lower in those who received FFR-guided complete revascularization compared to infarct-related PCI alone. The primary endpoint was further reduced in patients with at least one non-infarct-related stenosis ≥90% compared to those with <90%. FFR fractional flow reserve, STEMI ST-elevation myocardial infarction. Source: Lønborg, et al., 2017 [13]
In the Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction, COMPARE-ACUTE trial, 885 patients with STEMI and multivessel disease who had undergone primary PCI of the infarct-related artery were randomized to undergo FFR-guided complete revascularization of non-infarct-related coronary arteries or no revascularization of non-infarct-related arteries. Unlike DANAMI3-PRIMULTI, FFR of the non-infarct-related artery was done in the acute STEMI setting, during the time of primary PCI. At 1 year those who underwent FFR-guided complete revascularization of non-infarct-related arteries had a lower risk of death (1.4 vs. 1.7%), MI (2.4 vs. 4.7%), revascularization (6.1 vs. 17.5%), and cerebrovascular events (0.0 vs. 0.7%) compared with those who were treated for the infarct-related artery only [14]. Approximately half of the non-infarct-related artery lesions that were angiographically significant were not physiologically significant by FFR (≥0.80).
15.6 FFR in Other Acute Coronary Syndrome
FFR also plays a role in patients with non-STEMI ACS. This population may be more difficult to study than patients with STEMI because the culprit vessel is not always clear in NSTEMI or unstable angina (USA) group. The Impact of Routine Fractional Flow Reserve on Management Decision and 1-Year Clinical Outcome of Patients with Acute Coronary Syndrome (PRIME-FFR) pooled the results of the French FFR Registry (R3F) and the Portuguese Study on the Evaluation of FFR-Guided Treatment of Coronary Disease (POST-IT). A total of 1983 patients with NSTEMI or unstable angina (USA) were included in the large, international prospective study. They found that the use of FFR was associated with a high rate of reclassification of treatment. The percent of patients with ACS who were reclassified by FFR was similar to those with non-ACS (38 vs. 39%, P = NS). There was no significant difference in MACE (8.0 vs. 11.6%, p = 0.2) or symptoms (92.3 vs. 94.8%, p = 0.25) in patients reclassified based on FFR compared to those who were not reclassified. Fewer patients with ACS were reclassified from revascularization to medical treatment compared with those with non-ACS (P = 0.01). Furthermore, deferral to medical treatment based on FFR was as safe in ACS as it was in non-ACS patients. Patients with disregarded FFR had higher rates of MACE [15].
The ideal threshold for FFR in the ACS population has been debated. The FFR threshold of 0.80, which is used to determine functional significance in the stable ischemic heart disease (SIHD) population, was applied to the ACS population in the above studies. The accuracy of this threshold for ACS patients has been challenged. Hakeem et al. found that using the standard FFR threshold of 0.80 for clinical decision-making in ACS patients was associated with a threefold increase in the risk of subsequent MI and target vessel failure compared with SIHD patients and advised caution in using FFR-derived values for clinical decision-making in patients with ACS. They found that ACS patients had a higher FFR threshold of functional significance, and those with an FFR <0.85 had significantly higher event rates than those with FFR >0.85 [16].
Lee et al. found that regardless of the FFR value (FFR = 0.8–1.0), non-culprit lesions of ACS had a more than twofold higher rate of MACE that that of SIHD. The clinical outcomes of 449 non-culprit lesions in 301 patients with ACS were compared with the outcomes of lesions in patients with SIHD. The primary outcome of MACE (a composite of cardiac death, target vessel-related MI, and ischemia-driven revascularization) was higher in the ACS population (3.8 vs. 1.6%, HRadj 2.97, 95% CI: 1.23–7.17, p = 0.016) after 2 years and was mainly driven by a higher rate of ischemia-driven revascularization [17]. Table 15.1 summarizes the key trials of FFR in STEMI and other ACS.
15.7 Coronary Flow Reserve and the Index of Microvascular Resistance
Coronary flow reserve measures flow through both the epicardial resistance conduit and the microcirculation. It represents the vasodilator capacity of the coronary vascular bed during hyperemia and is another validated index of the functional significance of a coronary stenosis. Since most resistance occurs in the microcirculation, it is a primary method to measure microvascular flow. It is well-established that the failure to achieve myocardial reperfusion in a STEMI results in myocardial hemorrhage and infarct. Despite timely reperfusion therapy with PPCI, microvascular obstruction still occurs in up to 25–50% of STEMI patients who go on to have a higher degree of MACE. Therefore, measuring microvascular function may be a useful way to risk stratify STEMI patients.
In a STEMI, the microvasculature of the myocardium supplied by the non-infarct coronary arteries, in addition to the infarct-related artery, is abnormal. Cheng et al. evaluated 18 patients with acute MI and found that the CFR in the remote region (i.e., the region supplied by the non-infarct artery) was linked to CFR in the infarcted region and correlated with infarct size and severity. Even after successful PCI, the CFR in the myocardium supplied by the infarct-related artery was lower than normal [18]. A CFR > 2.0 is generally considered normal.
The index of microvascular resistance (IMR) is a direct invasive measure of microvascular resistance. It is defined as the distal coronary pressure multiplied by the mean transit time of a 3 mL bolus of saline at room temperature during maximal coronary hyperemia. It has been less well studied compared to FFR, but it may be a useful tool in evaluating the microcirculation during primary PCI. An IMR > 40 is considered abnormal and has been found to be associated with microvascular pathology, changes in LV function and EDV, and all-cause death and heart failure [19]. In a meta-analysis of six studies, a median IMR > 40 was an independent predictor of death [20]. In some studies, the combination of a high IMR and reduced CFR enhances the detection of microvascular obstruction, but in other studies, the combination did not have any prognostic value [21, 22].
An invasive measurement of the coronary microcirculation at the end of primary PCI may be a more sensitive measure of successful reperfusion compared with standard tests such as angiography or ECG. This could help identify high-risk patients who may benefit from continued treatment such as with glycoprotein IIb/IIIa inhibitors or intracoronary thrombolysis.
15.8 Instant Wave-Free Ratio
iFR is a resting index used to assess the severity of an intracoronary stenosis. It measures the ratio of the Pd to the Pa during an isolated period of diastole (i.e., the “wave-free period”). It is an attractive alternative to FFR because it does not require hyperemia and therefore has a lower incidence of patient discomfort, side effects, and a shorter procedural time. iFR has been shown to be non-inferior compared to FFR in two large, multicenter, randomized controlled trials: the Instantaneous Wave-Free Ratio Versus Fractional Flow Reserve in Patients with Stable Angina Pectoris or Acute Coronary Syndrome (iFR-SWEDEHEART) trial and the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization (DEFINE-FLAIR) trial. Both included patients with NSTEMI ACS, while DEFINE-FLAIR also included STEMI patients. The iFR-SWEDEHEART trial included 2037 patients with stable angina, unstable angina, or NSTEMI and randomly assigned them to undergo either iFR- or FFR-guided revascularization. For patients with unstable angina or NSTEMI, only non-culprit lesions were evaluated, and the culprit lesions were managed as clinically indicated. The rate of primary endpoint (composite of death from any cause, nonfatal MI, or unplanned revascularization within 12 months after the procedure) was not significantly different between the iFR and FFR groups [23].
In the DEFINE-FLAIR trial, 2492 patients with CAD were randomized to have iFR-guided or FFR-guided coronary revascularization. Patients with ACS were included but only non-culprit vessels and outside of primary intervention during acute STEMI. The primary endpoint of a composite of death from any cause, nonfatal MI, or unplanned revascularization did not differ significantly between groups. Additionally, the number of patients in the iFR group had lower rates of adverse side effects from the procedure (3.1 vs. 30.8%) and a shorter median procedural time (40.5 vs. 45.0 min) compared with the FFR group [24].
Conclusion
The physiological assessment of coronary stenoses is an integral part of the decision-making process for interventional cardiologists. Functional assessment in the STEMI and other ACS situation should consider the altered milieu of the microvasculature in an infarcted territory. While FFR is not valid in the infarct-related artery until recovery of the injured myocardium, the microcirculation in the non-infarct-related artery is not generally affected to reduce the accuracy of FFR and iFR. Future applications of translesional physiologic indices will expand the use of these methods into unique clinical scenarios to improve outcomes and prognosis.
References
Adjedj J, De Bruyne B, Floré V, et al. Significance of intermediate values of fractional flow reserve in patients with coronary artery disease. Circulation. 2016;133:502–8. https://doi.org/10.1161/CIRCULATIONAHA.115.018747.
Agarwal SK, Kasula S, Edupuganti MM, et al. Clinical decision-making for the hemodynamic “gray zone” (FFR 0.75–0.80) and long-term outcomes. J Invasive Cardiol. 2017;29(11):371–6.
Ahn JM, Park DW, Shin ES, et al. Fractional flow reserve and cardiac events in coronary artery disease: data from a prospective IRIS-FFR registry (interventional cardiology research Incooperation society fractional flow reserve). Circulation. 2017;135:2241–51. https://doi.org/10.1161/CIRCULATIONAHA.116.024433.
Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36:3182–8. https://doi.org/10.1093/eurheartj/ehv452.
Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. https://doi.org/10.1056/NEJMoa0807611.
De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. https://doi.org/10.1056/NEJMoa1205361.
Bodí V, Sanchis J, Núñez J, López-Lereu MP, Mainar L, Bosch MJ, et al. Abnormal myocardial perfusion after infarction in patients with persistent TIMI grade-3 flow. Only an acute phenomenon? Rev Esp Cardiol. 2007;60:486–92.
O’Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;61:e78–e140. https://doi.org/10.1016/j.jacc.2012.11.019.
Layland J, Rauhalammi S, Watkins S, et al. Assessment of fractional flow reserve in patients with recent non-ST-segment-elevation myocardial infarction: comparative study with 3-T stress perfusion cardiac magnetic resonance imaging. Circ Cardiovasc Interv. 2015;8:e002207. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002207.
Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–23. https://doi.org/10.1056/NEJMoa1305520.
Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–72. https://doi.org/10.1016/j.jacc.2014.12.038.
Engstrøm T. The third Danish study of optimal acute treatment of patients with ST-segment elevation myocardial infarction: Primary PCI in Multivessel disease. Presented at American College of Cardiology/i2 Scientific Session, San Diego, California, 16 March 2015.
Lønborg J, Engstrøm T, Kelbæk H, et al. Fractional flow reserve-guided complete revascularization improves the prognosis in patients with ST-segment-elevation myocardial infarction and severe nonculprit disease: a DANAMI 3-PRIMULTI substudy (primary PCI in patients with ST-elevation myocardial infarction and multivessel Disease: treatment of culprit lesion only or complete revascularization). Circ Cardiovasc Interv. 2017;10:e004460. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004460.
Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–44. https://doi.org/10.1056/NEJMoa1701067.
Van Belle E, et al. Impact of routine fractional flow reserve on management decision and 1-year clinical outcome of patients with acute coronary syndromes. Circ Cardiovasc Interv. 2017;10(6):e004296. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004296.
Hakeem A, Edupuganti MM, Almomani A, et al. Long-term prognosis of deferred acute coronary syndrome lesions based on nonischemic fractional flow reserve. J Am Coll Cardiol. 2016;68:1181–91. https://doi.org/10.1016/j.jacc.2016.06.035.
Lee JM, Choi KH, Koo BK, et al. Prognosis of deferred non-culprit lesions according to fractional flow reserve in patients with acute coronary syndrome. EuroIntervention. 2017;13(9):e1112–9.
Cheng R, et al. Coronary flow reserve in the remote myocardium predicts left ventricular remodeling following acute myocardial infarction. Yonsei Med J. 2014;55(4):904–11.
Carrick D, et al. Comparative prognostic utility of indices of microvascular function alone or in combination in patients with an acute ST-segment elevation myocardial infarction. Circulation. 2016;134(23):1833–47. https://doi.org/10.1161/CIRCULATIONAHA.116.022603.
Bulluck H, et al. Index of microvascular resistance and microvascular obstruction in patients with acute myocardial infarction. JACC. 2016;9:2172–3.
Park SD, Baek YS, Lee MJ, Kwon SW, Shin SH, Woo SI, Kim DH, Kwan J, Park KS. Comprehensive assessment of microcirculation after primary percutaneous intervention in ST segment elevation myocardial infarction: insight from thermodilution-derived index ofmicrocirculatory resistance and coronary flow reserve. Coron Artery Dis. 2016;27:34–9.
Ahn SG, Hung OY, Lee JW, Lee JH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Lee SH, Yoon J, Kwon W, Samady H. Combination of the thermodilution-derived index of microcirculatory resistance and coronary flow reserve is highly predictive of microvascular obstruction on cardiac magnetic resonance imaging after ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2016;9:793–801.
Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813–23. https://doi.org/10.1056/NEJMoa1616540.
Davies JE, Sen S, Dehbi HM, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–34. https://doi.org/10.1056/NEJMoa1700445.
Disclosures
MJK is a consultant and speaker for Abbott/St. Jude, Philips/Volcano, Acist Medical Inc., Opsens Inc., and HeartFlow Inc.
KMY has no conflict of interest to declare.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Yu, K.M., Kern, M.J. (2018). Physiological Lesion Assessment in STEMI and Other Acute Coronary Syndromes. In: Watson, T., Ong, P., Tcheng, J. (eds) Primary Angioplasty. Springer, Singapore. https://doi.org/10.1007/978-981-13-1114-7_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-1114-7_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1113-0
Online ISBN: 978-981-13-1114-7
eBook Packages: MedicineMedicine (R0)