Abstract
Bacterial infections are the most significant infectious source of morbidity and mortality in cirrhotic patients. Bacteria infections result is both acute decompensation in chronic liver disease and mortality in patients with decompensated cirrhosis. Spontaneous bacterial peritonitis (SBP), bacteremia, pneumonia, urinary tract infections (UTI) and skin and soft tissue infection (SSTI) are the most significant sources of infection in cirrhosis. Bacterial infections can precipitate renal failure and worsening hepatic encephalopathy, and patients with sepsis and liver disease have higher rates of acute respiratory distress syndrome (ARDS) and coagulopathy.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
Understand the impact of bacterial infections on cirrhosis.
-
Discuss the pathophysiologic mechanisms that put patients with cirrhosis at risk for bacterial infections.
-
Discuss the presentation, diagnosis, management and prevention of spontaneous bacterial peritonitis (SBP).
-
Describe the microbiology of bacteria associated with blood stream infections, pneumonia, skin and soft tissue infection (SSTI), and urinary tract infections in patients with chronic liver disease.
Bacterial infections are the most significant infectious source of morbidity and mortality in cirrhotic patients. At least one-third of hospitalized patients with cirrhosis will have a bacterial infection compared with less than 10% of otherwise healthy hospitalized patients [1,2,3]. Bacterial infections continue to be the leading cause of both acute decompensation in chronic liver disease and mortality in patients with decompensated cirrhosis, accounting for 30–50% of deaths [2, 4, 5]. Spontaneous bacterial peritonitis (SBP), bacteremia, pneumonia, urinary tract infections (UTI) and skin and soft tissue infection (SSTI) are the most significant sources of infection in cirrhosis. While recognition and treatment of SBP has decreased mortality from 80% to 20% in the past 30 years, mortality from infections, particularly pneumonia and septicemia, remain quite high [1]. Bacterial infections can precipitate renal failure and worsening hepatic encephalopathy, and patients with sepsis and liver disease have higher rates of acute respiratory distress syndrome (ARDS) and coagulopathy compared to patients with sepsis and no underlying liver disease [6, 7].
1 Pathophysiology of Bacterial Infection Risk in Cirrhosis
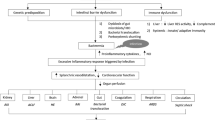
Historically, bacterial infections in patients with liver disease are primarily due to translocation of native gut bacteria complicated by immune dysregulation. In healthy persons, low-grade bacterial translocation occurs regularly, as bacteria travel to the liver though mesenteric lymph nodes and the portal venous system. The reticuloendothelial system of the liver, compromised of Kupffer cells, exert a filtering effect, inhibiting bacteria from reaching the systemic circulation [1, 6,7,8,9]. Animal models suggest that both damage to Kupffer cells as well as septal and sinusoidal fibrosis increase risk of bloodstream infections (BSI) and SBP [8]. Small bowel bacterial overgrowth and decreased intestinal motility in cirrhosis also contribute to bacterial translocation from the gut [7]. Proton-pump inhibitors, commonly prescribed for gastrointestinal bleeding in cirrhosis, alter the microbiome and appear to facilitate bacterial translocation as well [1, 9, 10].
Cirrhosis-associated immunodeficiency (CAID) describes the array of immunodeficiency present in cirrhosis that lead to the evolution of infections. The healthy liver produces complement proteins and Protein C, which play critical roles in the adaptive immune system’s effector response. Production of these key proteins are reduced in patients with cirrhosis [2, 7]. Splenomegaly leads to sequestration of circulating monocytes, neutrophils, and lymphocytes and further impairs cellular immunity [6]. Impaired phagocytosis and chemotaxis also contribute to the evolution of infection in cirrhosis [2, 6,7,8]. Malnutrition and alcohol use, which are common in cirrhotic patients, further compromise immune function [2, 5].
The systemic response to bacterial infections in cirrhosis is profound. Since cirrhotic patients have a baseline hyperdynamic circulatory state, infection often facilitates cardiovascular collapse and places patients at high risk of septic shock. Nitric oxide, which is a primary driver of systemic vasodilation and circulatory shock, may be overexpressed in cirrhotic patients [6, 7]. Upregulation of various cytokines, including TNF-α, IL-1, IL-6, and IL-17, also occurs in liver disease and contributes to exaggerated responses to infection [2, 6, 8]. Lastly, since the diseased liver is less efficient at clearing bacterial endotoxins, exaggerated systemic response to bacterial infections may occur more frequently [2, 6, 7].
2 Spontaneous Bacterial Peritonitis
Spontaneous Bacterial Peritonitis (SBP) is defined as infection of ascitic fluid in the absence of an intraabdominal source [11]. It is the most frequent bacterial infection in cirrhosis and accounts for 25–31% of bacterial infections in cirrhosis [7]. The mechanism of infection involves translocation of gastrointestinal bacteria to the portal vein and mesenteric lymph nodes and spillage into ascitic fluid [10,11,13]. Cirrhotic ascites is a primarily transudative fluid, with low opsonic activity [12], and, as a result, bacterial growth may proceed unabated. Ten percent of cirrhotic patients with ascites will developed SBP within 1 year of diagnosis, and SBP is present in 30–50% of all hospitalized patients with cirrhosis [7]. The development of SBP also predicts mortality—1-year mortality after the first episode of SBP is over 30% [7]. While SBP is classically associated with cirrhotic ascites, SBP is also present in patients with ascites secondary to acute liver failure [10].
2.1 Presentation
Although hypothermia is common among cirrhotic patients, fever, which may be mild, is one of the most common presenting symptoms in patients with SBP. Other symptoms include abdominal pain, increased amount of ascitic fluid, failure of diuretic treatment, new or worsening hepatic encephalopathy, and diarrhea [1, 7]. Ten to thirty percent of patients with SBP are asymptomatic [1]. The International Ascites Club recommends that SBP be considered in the following circumstances: all cirrhotic patients with ascites on admission to hospital; patients with ascites who develop signs of sepsis, hepatic encephalopathy, renal impairment or altered gastrointestinal motility; all cirrhotic patients with ascites and a gastrointestinal bleed.
2.2 Risk Factors
Risk Factors for SBP include:
-
Advanced cirrhosis: high MELD scores and Child-Pugh stage C disease [14].
-
Hyponatremia (serum sodium <125 mg/dL) [14].
-
Genetic polymorphisms in TLR2 and NOD2, receptors which recognize bacterial [1, 3, 7, 9, 10].
Use of non-selective beta-blockers may protect against SBP, but their use may increase the risk of circulatory collapse from SBP and may decrease transplant free survival [10].
2.3 Microbiology
Historically, gram-negative bacilli have been the major cause of SBP. However, microbial patterns have shifted to include more gram-positive cocci with broader use of antibiotics and invasive procedures over the past three decades. From 1971 to 1991, E. coli was the most prevalent source of SBP, present in 46% of cases. Other common sources included Streptoccocus (30%), Klebsiella (9%). Since 1998, E. coli has remained the most common organism associated with SBP, but now accounts for only one-third of cases [10]. Gram-positive cocci now comprise 25% of pathogens isolated in SBP, with Streptococcus and Enterococcus being the most prevalent. Twenty-five to thirty percent of these GPC-associated SBP cases occur in patients taking fluoroquinolone prophylaxis [5, 16]. Multi-drug resistant gram-negative bacilli, including extended spectrum β-lactamase (ESBL) producing E. coli, carbapenem-resistant Klebsiella pneumonie and Pseudomonas aeruginosa have been isolated in cases of health-care acquired SBP [16]. Enterococcus faecium and methicillin-resistance staphylococcus aereus (MRSA) have also been isolated in healthcare associated SBP [5]. Anaerobes remain a rare source. Pathogens associated with cases of SBP from 1998–2015 are summarized in Fig. 15.1 [10].
2.4 Diagnosis
Paracentesis is required to diagnose SBP. Diagnosis can be made by when either the absolute neautrophil (polymophonuclear cell, PMN) count in the ascitic fluid is >250 cell/mm3 or ascitic fluid culture is positive, although careful interpretation of positive cultures with low cell counts in needed. The absolute PMN count can be calculated by adjusting for red blood cell contaimination (absolute PMN = [total WBC × % PMNs] − [RBC/250]).
Although the gold standard for diagnosing SBP is the presence of positive ascitic fluid culture and many polymorphonuclear cells (>250 cells/mm3), cultures are negative frequently (up to 40%) [10]. This is known as culture-negative neutrophilic ascites (CNNA), and is a common varient of SBP that should be treated the same as culture-positve SBP. The absence of a postive fluid culture in CNNA may reflect low pathogen burden [10, 12] and use of PCR demonstrates that bacterial DNA are present at detectable levels in most cases [17]. Blood culture bottles should be inooculated at the bedside with at least 10 mL of ascitic fluid to increase culture yield [7, 10]. When ascites fluid culture is positive, a single pathogen is isolated in 90% of cases [10]. Urinary reagent strips that assess leukocyte esterase activity should not be used to diagnose SBP due to an unacceptable low positive predicitve value and high false negative rate [7, 10].Assays of lactoferrin in ascitic fluid may be a more accurate predictor of infection [3, 7].
Infrequently, secondary bacterial peritonitis resulting from a perforated viscus may be present when spontaneous bacterial peritonitis is suspected. Polymicrobial organisms on a gram stain are indicative of secondary bacterial peritonitis. Additionally, analysis of asctitc fluid chemistry using Runyon’s criteria can be used to differentiate SBP from secondary bacterial peritonitis. The presence of two or more of the these criteria are 90% specific for secondary bacterial peritonitis [18]:
-
1.
Total protein >1 g/dL
-
2.
Glucose <50 mg/dL
-
3.
LDH > upper limit of normal for serum
2.5 Management
Community-acquired SBP should be treated with antibiotics that cover Enterobacteriaceae and non-enterococcal gram-positve cocci. Intravenous antibiotics, including a third-generation cephalosporin or amoxicillin-clavulante are recommended; IV amoxacillin-clavulanate is not available in the United States. Cefotaxime has been the most well studied, and has excellent penetration into ascitic fluid [10]. Ceftriaxone is also effective in treating SBP, though its high protein-binding activity makes it theoretically less effective in cirrhosis, where protein synthesis is impaired [10]. Oral ofloxacin and IV ciprofloxacin may also be used in uncomplicated SBP, but should be avoided in patients on fluoroquinalone prophylaxis or in geographic areas with high levels of fluoroquinalone resistance to Enterobacteriaceae [7, 19]. Levofloxacin may be efficacious in patients on fluorquinalone prophyalxis who cannot tolerate β-lactams [10]. Treatment should be continued for 5 days, as 5 day courses of antibiotics are as efficacious but less costly than 10 day courses [6, 10, 20]. Nosocomial infections, defined as infections occuring after 48 h of hospitalization, are be resistant to β-lactam in 33–78% of cases [5]. Meropenam or tigecycline can be used in nosocomial cases of SBP in areas with a high prevalence of ESBL-producing pathogens [3, 5, 7]. Antibiotic therapy should be tailored if culture is positive and sensitivies are available. Follow-up paracentesis 48 h after initiaition of treament is recommended in patients who do not rapidly improve [10]. A reduction in PMN count by >25% suggests appropriate antibiotic coverage; if such a reduction is not observed, antibiotics should be broadened or the possibility of secondary bacterial peritonitis should be considered [3, 7, 10].
Hepatorenal syndrome (HRS) is a common complication of infections in patients with cirrhosis, and occurs in 30–40% of cases of SBP [7]. The addition of albumin, which acts as a plasma expander, to antibiotic treatment significantly reduces the rate of HRS [3, 7, 10]. Treatment with albumin (1.5 g/kg on day 1 followed by 1 g/kg on day 3), is particularly effective and recommended for patients at high risk of HRS, identified as having any one of the following either bilirubin >4 mg/dL or creatinine >1 mg/dL [3, 7, 10]. Albumin is not necessary in patients who are at a low risk for HRS [3, 7].
2.6 Prevention
Patients who have a primary episode of SBP have a 40–70% chance of recurrent SBP within 1 year of initial SBP presentation [10]. Long-term use of norfloxacin has been shown to decreased the recurrence of SBP and is recommended as secondary prophylaxis for patients who have one episode of SBP; unfortunately, norfloxacin is not currently widely available commercially [6, 7, 19]. Ciprofloxacin and trimethorprim-sulfamethoxazole may also be used [10]. Daily dosing is recommended to limit growth of fluoroquinolone-resistant organisms [10]. Primary prophylaxis is also used in patients with low ascitic albumin concentration and GI bleeding, as these patients are at a particularly high risk for developing SBP [2, 5,6,7, 10]. The antibiotic used, route of administration, and length of treatment depends upon the indication for prophylaxis as outlined in Table 15.1.
3 Other Bacterial Infections
3.1 Bloodstream Infections
Bloodstream infections occur in 4–21% of cirrhotic patients, and are ten times more prevalent in patients with cirrhosis than in the general population [8]. Gut translocation is the primary mechanism of bacteremia in liver disease, and bacteria that reside in the gut—gram-negative bacilli, anaerobes, and Enterococcus—are the primary pathogens [6,7,8]. In the early 2000s, health-care acquired gram-positive cocci, including MRSA, became a common source of blood stream infections. Fluoroquinolone prophylaxis and increased use of broad-spectrum antibiotics have led to emergence of multi-drug resistant (MDR) and extreme-drug resistant (XDR) gram-negative bacilli. In a single-center study of 162 cirrhotic patients with a bloodstream infection, 60% of pathogens were resistant to third-generation cephalosporins. Of the gram-negative bacilli isolated, 25% were classified as MDR and 21% were classified as XDR [21].
3.2 Endotipsitis
Endotipsitis , ongoing bacteremia in the presence of an infected thrombus or endovascular infection affecting the TIPS, has been increasingly reported over the past 10 years, with Enterococcus and Staphylococcus as the most common isolated pathogens [8, 22]. Because a TIPS cannot be removed outside of liver transplantation, diagnosis and treatment of endotipsitis is more complicated than that of infections associated with removable indwelling devices. Endotipsitis should be suspected in patients with a TIPS and bacteremia when the source of bacteremia is unknown despite evaluation for other causes. If endotipsitis is suspected, patency of the TIPS should be evaluated using Doppler ultrasound—a thrombus or vegetation is strongly suggestive of endoptipsitis [22]. Treatment relies solely on the use of antibiotics, as source control with TIPS removal is not possible. There are no clear guidelines for the duration of treatment, though in case series, patients who successful cleared their bacteremia were treated for a mean duration of 6 weeks [22]. If bacteremia is prolonged or recurs after a prolonged period of therapy, chronic suppressive therapy until transplantation should be considered. Though active infection is often a contraindication for liver transplantation, there are reports of successful clearance of endotipsitis after the device is removed during transplant [22].
3.3 Pneumonia
Bacterial pneumonia is also a significant source of morbidity and mortality in cirrhotic patients. The epidemiology of community-acquired lower respiratory infections is the same in patients with and without liver disease with Streptococcus pneumoniae being the most common cause. Other common causes include oropharyngeal bacteria, including Haemophilus influenza and anaerobes as well as less common bacteria, including Klebsiella, Legionella and Mycoplasma [1, 2]. Pseudomonas aeruginosa is one unique bacteria that is more commonly identified in patients with liver disease than those without [23]. The severity of pneumonia in patients with chronic liver disease is enhanced with a higher rate of ICU admission, more severe clinical presentations, increased prevalence of bacteremia and increased mortality [1, 9, 23]. Pneumococcal vaccination is recommended for adults with chronic liver disease; while the 23-valent polysaccharide pneumococcal (PPSV-23) vaccine is primarily recommended for this patient population, those individuals who are being evaluated for transplantation should also receive the 13-valent pneumococcal conjugated vaccine (PCV13) [24]. Ideally, the PCV13 should be given first followed by the PPSV-23 6 months or more after the PCV13 vaccine.
3.4 Skin and Soft Tissue Infections (SSTIs )
Chronic liver disease also predisposes patients to skin and soft tissue infection. Venous insufficiency, lower extremity edema and immune dysfunction predispose chronic liver disease patients to skin infections of the lower extremity. While gram-positive cocci are the most common source of SSTIs in liver disease [1, 2], gram-negative bacilli are also relatively common in patients with liver disease compared to controls. A prospective study in India identified gram-negative bacilli as the most common isolate in cirrhosis, with male sex, alcohol use, and bare-foot walking being major risk factors [25]. Vibrio vulnificans, a rare, curved gram-negative bacillus found in warm seawater, can invade open wounds and cause severe hemorrhagic bullae and rapid necrotizing fasciitis. Vibrio vulnificans is also associated with bacteremia and septic shock after consumption of oysters grown in infected waters. In a study of over 1000 cases in Japan, 23% of patients with Vibrio vulnificans had underlying cirrhosis [26]. Cirrhosis due to hemochromatosis is a particularly strong risk factor for Vibrio infection [7].
3.5 Urinary Tract Infection (UTI)
Urinary Tract Infections are common in cirrhosis, particularly in females [2]. Bacteruria is often asymptomatic, and indwelling catheters are a significant risk factor [2]. Recurrent UTI, most often with E. coli is often found in patients with primary biliary cirrhosis (PBC), and to a lesser extent in autoimmune hepatitis (AIH), even prior to diagnosis [27]. It has been hypothesized that molecular mimicry between a human and E. coli epitope may account for this phenomenon [27].
4 Clostridium Difficile Infection and C. difficile Associated Diarrhea (CDAD)
Clostridium Difficile infection resulting in diarrhea is becoming increasingly common and cirrhotic patients are more likely to contract CDAD than the general population [7, 28]. C. difficile is more common among hospitalized patients with liver disease than among those without liver disease; alcoholic hepatitis and autoimmune hepatitis are particularly strong risk factors for CDAD [28]. Risk factors for CDI in cirrhosis are similar to risk factors in the general population and include antibiotic use, including outpatient fluoroquinolone prophylaxis, and PPI use [7, 28, 29]. Fluoroquinolone prophylaxis for SBP use is associated with infection with the particularly virulent NAP1 strain [28]. Cirrhotic patients with CDAD have longer lengths on hospital stay and increased mortality compared with cirrhotic patients without CDAD [7, 28, 29].
4.1 Management of CDAD
Treatment of C. difficile in patients liver disease is similar to treatment in patients without liver disease, and depends on disease severity. CDI/CDAD severity can be divided into three categories [30]:
-
1.
Mild to moderate, which involves with diarrhea and absence of any features of severe or severe and complicated disease.
-
2.
Severe, which includes a serum albumin of less than 3 g/dL AND either a white blood cell count >15,000 cells/mm3 OR abdominal tenderness.
-
3.
Severe and complicated, defined as CDAD/CDI in patients with a fever >38.5 °C, WBC >35,000 cells/mm3 or <2000 cells/mm3, those who require admission to an intensive care unit, have evidence of shock, including hypotension requiring vasopressors, lactate >2.2 mmol/L, altered mental status, or other end organ damage.
The first line treatment for mild to moderate CDI/CDAD consists of oral metronidazole (or IV metronidazole in patients who are unable to take medication by mouth) for 10–14 days. Oral vancomycin is traditionally reserved for cases refractory to metronidazole or for severe CDI/CDAD. Patients with severe and complicated CDI should be treated with both oral vancomycin and intravenous metronidazole [28, 30, 31]. Vancomycin can be administrated rectally if ileus is present, and surgery should be considered in severe refractory disease. Treatment with fidoximicin for recurrent CDI has been proven effective in the general population, but there is no data specific to patients with liver disease [28].
Summary Learning Points
-
Bacterial infections are the leading cause of mortality in chronic liver disease.
-
Translocation of gut bacteria, particularly gram-negative bacilli, occurs frequently in cirrhosis and is the major mechanism putting this population at risk for bacterial infection.
-
Cirrhosis acquired immunodeficiency (CAID) is a collection of immune system deficiencies in both innate and adaptive immunity in cirrhosis.
-
Spontaneous bacterial peritonitis (SBP) is the most frequent bacterial infection seen in cirrhosis. Risk factors include advanced cirrhosis, GI bleeding, low ascitic protein count, and PPI use. All patients with cirrhosis admitted to the hospital should undergo diagnostic paracentesis regardless of reason for admission.
-
SBP should be treated with a third-generation cephalosporin. Secondary prophylaxis should be given after the first episode of SBP. Primary prophylaxis should be given in patients with GI bleeds and low ascitic protein count.
-
Primary bacteremia is also a significant cause of morbidity in cirrhosis. Recently, there has been an increase in prevalence of in gram-positive cocci and multi-drug or extreme-drug resistant gram-negative bacilli.
-
Bacterial pneumonia can be particularly severe in cirrhosis. All patients with chronic liver disease should be vaccinated with PPSV-23.
-
Skin and Soft Tissue infections (SSTIs) in cirrhosis are often due to the same gram-positive cocci that cause SSTI in healthy persons. However, cirrhosis greatly increases the risk of infection with gram-negative bacilli and the particularly virulent Vibrio vulnificans pathogen.
-
Urinary Tract Infections are common and may be asymptomatic in cirrhosis.
-
Clostridium Difficile associated diarrhea is more common in cirrhotic patients compared to the general population and is associated with poor outcomes in cirrhosis.
5 Viral Infections in Chronic Liver Disease
5.1 Hepatitis A Virus
Hepatitis A virus (HAV) accounts for half of all causes of viral hepatitis in the United States [32]. The illness begins with a period of nausea and anorexia and progresses to an icteric phase with jaundice and a marked elevation in bilirubin [33]. The disease is usually resolves and rarely results in acute liver failure or death. However, the disease is significantly more severe in chronic liver disease. Patients with underlying chronic liver disease have up to a 23-fold increase in mortality compared to patients with hepatitis A and no underlying liver disease [34]. Those with underlying HCV infected with HAV have higher mortality rates and are more likely to develop acute liver failure compared with individuals with underlying HBV or no underlying viral liver disease at the time of HAV infection [32, 34, 35]. The hepatitis A vaccine is both safe and effective in patients with chronic liver disease, and it is recommended for all patients with chronic liver disease [35]. However, the vaccine is less immunogenic in patients with advanced liver disease, suggesting vaccination early after the diagnosis of liver disease [34].
5.2 Hepatitis B Virus
Hepatitis B virus can be the underlying cause of chronic liver disease or occur in individuals with underlying liver disease due to other causes. Vaccination against hepatitis B is of particular importance in patients with liver disease listed for liver transplant, as new active infection can occur post-transplant in non-immune recipients from donor livers from chronic, HBsAg-negative, carriers. Like the HAV vaccine, the HBV vaccine has good immunogenicity in mild to moderate chronic liver disease, but poor immunogenicity in end stage liver disease [34]. The impact of superimposed HBV on chronic liver disease has primarily been studied in the cohort of patients with HCV and is discussed below. However, given the severity of superimposed HBV infection, HBV vaccination is recommended in all patients with ESLD.
5.3 Hepatitis B and Hepatitis C Co-infection
Hepatitis B and C share similar risk factors and commonly occur in the same individual. Between 2 and 10% of patients with hepatitis C virus (HCV) have a detectable hepatitis B surface antigen (HBsAg) [36]. However, high sensitivity testing for HBV DNA can detect occult hepatitis B infection in up to one-third of HCV patients with undetectable HBsAg [37], suggesting the incidence of co-infection is under-recognized [36]. It is thought that HCV exerts a suppressive effect on HBV, because HBV DNA levels are relatively low in HBV/HCV coinfection compared to HBV monoinfection [36, 38]. The most common scenario in which HCV/HBV coinfection occurs is a HCV superinfection on chronic HBV infection [36]. Fulminant hepatic failure due to HCV alone is rare; however studies from areas of where hepatitis B is endemic suggest that chronic underlying HBV at the time of acute HCV infection results in a sevenfold increases in the risk of fulminant hepatic failure [36, 39]. HBV acquisition in pre-existing hepatitis C is less common, but has been reported to result in the development of ascites and hepatic encephalopathy [36]. Coinfection of HBV/HCV results in substantially higher rates of progression to both cirrhosis and hepatocellular carcinoma compared with monoinfection with either virus [36, 38, 40].
5.4 Hepatitis D Virus
Hepatitis D virus (HDV) is an RNA virus with tropism for hepatocytes and relies on HBsAg for survival. Therefore, infection with HDV occurs only in individuals with HBV infection. Two infection patterns are observed:
-
1.
Co-infection occurs in acute HBV infection when HDV infects the same individual at the same time. The course mimics acute HBV infection, though HDV co-infection is a risk factor for progression to fulminant hepatitis [41]. Because the majority of acute hepatitis B episodes are self-limited and result in disappearance of HBsAg and appearance of anti-HBsAg antibodies, HDV disappears once seroconversion occurs.
-
2.
Superinfection occurs when HDV infects a chronic HBsAg-positive carrier, resulting in a particularly virulent acute hepatitis or decompensation [41]. Half of all cases of acute liver failure in HBsAg-positive individuals occurs in the presence of HDV [41]. HDV persists in 90% of cases of superinfection and leads to cirrhosis within 5–10 years in 70% of cases [41].
5.5 Hepatitis E Virus
Hepatitis E virus (HEV) causes an acute hepatitis similar to hepatitis A and is primarily found in Asia, Africa and the Middle East. Studies from endemic regions of the world suggest that HEV causes rapid decompensation in cirrhosis with mortality as high as 70% at 4 weeks post-infection [42].
5.6 Human Immunodeficiency Virus (HIV )
In the era of antiretroviral therapy, liver disease is the most common cause of death in HIV-infected individuals, accounting for 14–18% of all deaths [43]. Coinfection of HIV in chronic viral hepatitis is common.
In the United States and Europe, 30% of HIV patients are co-infected with HCV [43]. Shared risk factors include injection drugs use and exposure to blood products. Hemophiliacs with HIV have a 60–90% risk of coinfection with HCV, and HIV positive injection drug users have a 50–90% HCV coinfection rate [44, 45]. HIV coinfection with HCV halves the likelihood of clearing HCV viremia, and accelerates the progression to cirrhosis [43, 44]. Decompensated cirrhosis is 2–6 times more common in HCV cirrhosis when HIV infection is present [43, 44]. Treatment of HIV with anti-retroviral therapy (ART) reduces, but does not completely eliminate, the impact of HIV infection on HCV disease progression [45]. Treatment of HCV with interferon/ribavirin is more effective at higher CD4 counts, and thus ART for HIV should be initiated prior to treatment of HCV with interferon/ribavirin if CD4 count is <500/μL [45]. New direct-acting antivirals (DAAs) for the treatment of HCV are expected to eliminate the need to elevate CD4 count prior to HCV treatment, but careful consideration of drug interactions between DAAs and ARTs is necessary when treating both infections [46].
Approximately 10% of HBV infected individuals are co-infected with HIV [43]. HIV increases the risk of developing chronic HBV infection, with a more profound effect at lower CD4 counts [43, 47]. HIV also enhances the progression to cirrhosis and increases the risk of developing hepatocellular carcinoma in HBV infection [47]. Much of the hepatocellular toxicity in hepatitis B is due to immune response against hepatic cells, which would imply that hepatocellular toxicity should be diminished when a virus causing immunodeficiency is present. Indeed, HIV/HBV coinfection results in lower alanine aminotransferase (ALT) levels compared to HBV monoinfection [47]. However, ALT levels do not correlate with clinical severity and liver biopsy is recommended to determine extent of disease [43, 47]. One mechanism of HIV-induced HBV exacerbation is that particularly virulent HBV strains are more prevalent in HIV-coinfected cases. A strain of HBV with a direct cytopathic effect, resulting from a deletion in the core/pre-core region of the HBV genome, has been shown to be more prevalent in HBV/HIV coinfection compared to HBV monoinfection [47]. HIV also induces microbial translocation, which may lead to increased immune activation and thus increased HBV-induced hepatocellular injury [47]. In general, when selecting anti-retroviral therapy (ART) for HIV treatment, agents that also have activity against HBV should be used—tenofovir plus emtricitabine or tenofovir plus lamivudine are common regimens [43, 47]. Treatment of HIV without appropriately treating HBV can rarely cause worsening of hepatitis due to immune reconstitution inflammatory syndrome (IRIS) [43, 47]. Further, interruption of HBV-active anti-retrovirals may cause an acute hepatitis B reactivation and rapid progression of liver disease.
5.7 Influenza Infection
Data regarding the effect of influenza virus in cirrhosis is largely limited to case reports. Influenza A (H3N2) was associated with three cases of decompensated cirrhosis during the 1997–1998 epidemic [48]. In a small, single-center case series, influenza A/H1N1/09 was associated with lethal ARDS and pneumonia in patients with cirrhosis [49]. Influenza vaccine has good immunogenicity in cirrhotic patients, with trends toward a reduction in influenza infection and hepatic decompensation in vaccinated cirrhotic patients [40]. Patients with cirrhosis who develop influenza infection should be treated with active therapy, including one of the neuraminidase inhibitors, oseltamivir, peramivir or zanamivir, as soon as influenza is suspected without waiting for results of testing to confirm infection.
Summary Learning Points
-
Hepatitis A and B vaccines should be administered to all patients with chronic liver disease.
-
HBV and HCV frequently co-exist and are associated with increased rates of cirrhosis compared to monoinfection with either virus.
-
HIV is commonly present in individuals infected with either HBV or HCV, and increases the severity of liver disease in these patients.
-
Cirrhosis predisposes to severe pulmonary complications of influenza infection. These patients should receive the influenza vaccine to prevent infection and early treatment with neuraminidase inhibitors when infection is suspected.
6 Infection in Acute Liver Failure
Acute liver failure (ALF) in the absence of known, pre-existing liver disease is relatively rare, with about 2000 cases per year in the United States [50], and thus is not as well studied as acute decompensation of cirrhosis. Patients with acute liver failure, like those with cirrhosis, are at an increased risk of infection due to the impaired innate immunity and exposure to indwelling lines, and up to 90% of ALF patients will develop an infection while hospitalized [50]. However, the role of gut translocation in predisposition to infection is less defined in ALF [50, 51]. Infection most commonly develops early in the hospital course, within 2–5 days of admission, but may also develop after hospital day 10 and accounts for 25% of late mortality in ALF [50]. Because active infection is a contraindication to liver transplant, which is the only curative option in advanced ALF, infection has a significant indirect effect on mortality. Identifying infection in ALF may be difficult, as leukocytosis and fever are absent in 30% of cases [50].
Bacterial infections have been documented in up to 90% of patients with ALF. Historically, gram-positive cocci due to pneumonia predominated as the leading cause of blood stream infections in ALF [50]. More recently, gram negative organisms are becoming increasingly common and now account for close to half of bacterial infections with Klebsiella spp. being the most common gram-negative pathogen [50]. Fungal infections, including Candida, Aspergillus and Pneumocystis jiroveci may also occur in ALF [50, 51], particularly when renal dysfunction is present [51], and should be considered when leukocytosis or fever persist despite broad spectrum antibiotics. Reactivation of CMV infection has also been described in ALF, particularly in patients being treated with corticosteroids for the underlying cause of acute liver failure [50].
Retrospective review of over 200 patients in a liver intensive therapy unit showed that high grade hepatic encephalopathy and SIRS criteria on hospital admission were predictive of the development of bacteremia, and bacteremia was associated with increased need for mechanical ventilation and renal replacement therapy [50, 52, 53]. Oral antibiotics with poor absorption to decontaminate the bowel was once thought to decrease incidence of bacterial infection [50], but initiation of prophylactic antibiotics, including systemic antibiotics, has not been shown to decrease 21-day mortality [50, 54]. While antimicrobial prophylaxis has been shown to increases the likelihood of transplant in ALF due to acetaminophen overdose, it does not increase survival in this group [54]. Thus, routine antimicrobial prophylaxis in ALF is not recommended [50, 55]. However, routine surveillance with chest radiographs, fungal and bacterial cultures of blood, sputum and urine is recommended given the high incidence of infection in this population [55], and antibiotics should be started at the first sign of rapid clinical deterioration, especially when worsening hepatic encephalopathy develops [55].
Summary Learning Points
-
Infection occurs in up to 90% of patients with acute liver failure.
-
While routine prophylactic antibiotics is not recommended in ALF, surveillance for infection should occur regularly.
-
Antibiotics should be started in the absence of documented infection in patients with rapid clinical decline and severe hepatic encephalopathy.
References
Strauss E. The impact of bacterial infections on survival of patients with decompensated cirrhosis. Ann Hepatol. 2013;13(1):7–19.
Taneja SK, Dhiman RK. Prevention and management of bacterial infections in cirrhosis. Int J Hepatol. 2011;2011:784540.
Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56(Suppl 1):S1–12.
Bar K, Wisplinghoff H, Wenzel RP, Bearman GM, Edmond MB. Systemic inflammatory response syndrome in adult patients with nosocomial bloodstream infections due to enterococci. BMC Infect Dis. 2006;6:145.
Pleguezuelo M, Benitez JM, Jurado J, Montero JL, de la Mata M. Diagnosis and management of bacterial infections in decompensated cirrhosis. World J Hepatol. 2013;5(1):16–25.
Nanchal RS, Ahmad S. Infections in liver disease. Crit Care Clin. 2016;32(3):411–24.
Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: a critical review and practical guidance. World J Hepatol. 2016;8(6):307–21.
Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence. 2016;7(3):309–19.
Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20(10):2542–54.
Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis—bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41(11):1116–31.
Runyon B. Spontaneous bacterial peritonitis in adults: treatment and prophylaxis. In: Post T, ed. Up to date. Up to date, Waltham, MA. Accessed 23 July 2016.
Mowat C, Stanley AJ. Review article: Spontaneous bacterial peritonitis—diagnosis, treatment and prevention. Aliment Pharmacol Ther. 2001;15(12):1851–9.
Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350(16):1646–54.
Schwabl P, Bucsics T, Soucek K, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015;35(9):2121–8.
Xu HB, Wang HD, Li CH, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res. 2015;14(3):7490–501.
Alexopoulou A, Papadopoulos N, Eliopoulos DG, et al. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33(7):975–81.
Such J, Francés R, Muñoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36(1):135–41.
Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol. 2010;52(1):39–44.
Casper M, Mengel M, Fuhrmann C, et al. The INCA trial (impact of NOD2 genotype-guided antibiotic prevention on survival in patients with liver cirrhosis and ascites): study protocol for a randomized controlled trial. Trials. 2015;16:83.
Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991;100(6):1737–42.
Bartoletti M, Giannella M, Caraceni P, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. 2014;61(1):51–8.
Mizrahi M, Adar T, Shouval D, Bloom AI, Shibolet O. Endotipsitis-persistent infection of transjugular intrahepatic portosystemic shunt: pathogenesis, clinical features and management. Liver Int. 2010;30(2):175–83.
Viasus D, Garcia-Vidal C, Castellote J, et al. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore). 2011;90(2):110–8.
Fakhraei H, Khalilzadeh S, Khanbabaei G, et al. Current recommendations for pneumococcal vaccination of children and adults. Tanaffos. 2015;14(3):161–4.
Sood A, Midha V, Goyal O, et al. Skin and soft tissue infections in cirrhotics: a prospective analysis of clinical presentation and factors affecting outcome. Indian J Gastroenterol. 2014;33(3):281–4.
Nagao Y, Matsuoka H, Seike M, et al. Knowledge of Vibrio vulnificus infection among Japanese patients with liver diseases: a prospective multicenter study. Med Sci Monit. 2009;15(10):PH115–20.
Smyk DS, Bogdanos DP, Kriese S, Billinis C, Burroughs AK, Rigopoulou EI. Urinary tract infection as a risk factor for autoimmune liver disease: from bench to bedside. Clin Res Hepatol Gastroenterol. 2012;36(2):110–21.
Trifan A, Stoica O, Stanciu C, et al. Clostridium difficile infection in patients with liver disease: a review. Eur J Clin Microbiol Infect Dis. 2015;34(12):2313–24.
Bajaj JS, Ananthakrishnan AN, Hafeezullah M, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: a national and tertiary center perspective. Am J Gastroenterol. 2010;105(1):106–13.
Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98. quiz 499
Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;373(3):287–8.
Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286–90.
McIntyre N. Clinical presentation of acute viral hepatitis. Br Med Bull. 1990;46(2):533–47.
Keeffe EB. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006;117:227–37. discussion 237–8
Almasio PL, Amoroso P. HAV infection in chronic liver disease: a rationale for vaccination. Vaccine. 2003;21(19–20):2238–41.
Chu CJ, Lee SD. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol. 2008;23(4):512–20.
Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341(1):22–6.
Liu CJ, Chen PJ, Chen DS. Dual chronic hepatitis B virus and hepatitis C virus infection. Hepatol Int. 2009;3(4):517–25.
Chu CM, Yeh CT, Liaw YF. Fulminant hepatic failure in acute hepatitis C: increased risk in chronic carriers of hepatitis B virus. Gut. 1999;45(4):613–7.
Leise MD, Talwalkar JA. Immunizations in chronic liver disease: what should be done and what is the evidence. Curr Gastroenterol Rep. 2013;15(1):300.
Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis. 2012;32(3):228–36.
Kumar Acharya S, Kumar Sharma P, Singh R, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46(3):387–94.
Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8(12):1002–12.
Deng LP, Gui XE, Zhang YX, Gao SC, Yang RR. Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. World J Gastroenterol. 2009;15(8):996–1003.
Clausen LN, Lundbo LF, Benfield T. Hepatitis C virus infection in the human immunodeficiency virus infected patient. World J Gastroenterol. 2014;20(34):12132–43.
Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–54.
Thio CL, Hepatitis B. Human immunodeficiency virus coinfection. Hepatology. 2009;49(5 Suppl):S138–45.
Duchini A, Viernes ME, Nyberg LM, Hendry RM, Pockros PJ. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000;160(1):113–5.
Marzano A, Marengo A, Ruggiero T, et al. Clinical impact of A/H1/N1/09 influenza in patients with cirrhosis: experience from a nosocomial cluster of infection. J Med Virol. 2013;85(1):1–7.
Donnelly MC, Hayes PC, Simpson KJ. Role of inflammation and infection in the pathogenesis of human acute liver failure: clinical implications for monitoring and therapy. World J Gastroenterol. 2016;22(26):5958–70.
Craig DG, Lee A, Hayes PC, Simpson KJ. Review article: the current management of acute liver failure. Aliment Pharmacol Ther. 2010;31(3):345–58.
Karvellas CJ, Pink F, McPhail M, et al. Predictors of bacteraemia and mortality in patients with acute liver failure. Intensive Care Med. 2009;35(8):1390–6.
Arai M, Kanda T, Yasui S, et al. Opportunistic infection in patients with acute liver failure. Hepatol Int. 2014;8(2):233–9.
Karvellas CJ, Cavazos J, Battenhouse H, et al. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol. 2014;12(11):1942–1949.e1941.
Lee WMLA, Stravitz RT. AASLD position paper: the management of acute liver failure: update. 2011.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ison, M.G., Heldman, M. (2018). Bacterial Infections. In: Nanchal, R., Subramanian, R. (eds) Hepatic Critical Care . Springer, Cham. https://doi.org/10.1007/978-3-319-66432-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-66432-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66431-6
Online ISBN: 978-3-319-66432-3
eBook Packages: MedicineMedicine (R0)