Abstract
Background: Duration of treatment in tuberculosis of spine has always been debatable in the absence of marker of healing. The objective of the study was to evaluate the efficacy of extended DOTS regimen (2 months of intensive phase and 6 months of continuation phase) as recommended by WHO, by using MRI observations as the healing marker.
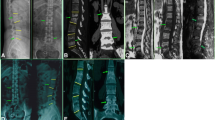
Materials and Methods: 51 (Group A -28 prospective and Group B- 23 retrospective) patients of spine TB with mean age of 26.8 years (range 15–54 years) diagnosed clinico radiologically/imaging (n=36), histopathology or by PCR (n=15) were enrolled for the study. They were treated by extended DOTS regimen (2 months of HRZE and 6 months of HR) administered alternate day. The serial blood investigations and X-rays were done every 2 months. Contrast MRI was done at the end of 8 months and healing changes were recorded. Criteria of healing on the basis of MRI being: complete resolution of pre and paravertebral collections, resolution of marrow edema of vertebral body (VB), replacement of marrow edema by fat or by calcification suggested by isointense T1 and T2 weighted images in contrast enhanced MRI. Patients with non healed status, but, responding lesion on MRI after 8 months of treatment were continued on INH and rifampicin alternate day and contrast MRI was done subsequently at 12 months and 18 months till the healed status was achieved.
Results: 9 patients had paraplegia and required surgical intervention out of which 1 did not recover neurologically. All patients have completed 8 months of extended DOTS regimen, n=18 achieved healed status and duration of treatment was extended in rest (n=33) 5 were declared healed after 12 months, 8 after 18 months and one after 36 months of treatment, thus 32 were declared healed at varying periods.
Conclusion: 35.2% patients demonstrate MRI based healed vertebral lesion at the end of 8 months of extended category 1 DOTS regimen. It is unscientific to stop the ATT by fixed time frame and MRI evaluation of the patients is required after 8 months of ATT and subsequently to decide for the continuation stoppage of treatment.
Similar content being viewed by others
References
Jain AK. Tuberculosis of spine: A fresh look at an old disease. J Bone Joint Surg Br 2010;92:905–13.
Global Tuberculosis Control: WHO Report 2010. p. 1–7.
Treatment of tuberculosis: guidelines. 4th ed. Geneva: WHO; 2010. p.95-8.
Rajeswari R, Balasubramaniam R, Venkatesan P. Short course chemotherapy in treatment of pott’s paraplegia. Int J Tuberc Lung Dis 1997;1:152–8.
Parthasarthy R, Sriram K, Santha T. Short course chemotherapy for tuberculosis of spine- a comparison between treatment and radical surgery. J Bone Joint Surg Br 1999;81:464–71.
Guo LX, Ma YZ, Chen X, Bao D, Luo XB. Clinical study of short course chemotherapy in spinal tuberculosis. Zhogguo Gu Shang 2010;23:491–4.
Tuli SM. Result of treatment of spinal tuberculosis by “middle path” regime. J Bone Joint Surg Br 1975;57:13–23.
Kotil K, Alan MS. Medical management of pott disease in thoracic and lumbar spine: A prospective clinical study. J Neurosurg Spine 2007;6:222–8.
Park K. Park’ textbook of preventive and social medicine. 18th ed. (2008) Jabalpur, India: Banarasidas Bhanot Publishers; p. 146–61.
A controlled trial of ambulant out-patient treatment and in patient rest in bed in the management of tuberculosis of the spine in young Korean patients on standard chemotherapy. J Bone Joint Surg Br 1973;55:678–97.
A controlled trial of plaster of paris jacket in the management of ambulant outpatient treatment of tuberculosis of the spine in children on standard chemotherapy. A study in Pusan. Tubercle 1973;54:261–82.
A controlled trial of debridement and ambulant treatment in the management of tuberculosis of the spine in patient on standard chemotherapy in Bulawayo, Rhodesia. J Trop Med Hyg 1974;77:72–92.
A controlled trial of anterior spinal fusion and debridement in the surgical management of tuberculosis of the spine in patient on standard chemotherapy. A study in Hong Kong. Br J Surg 1974;61:853–66.
A five years assessment of controlled trial of inpatient and outpatient treatment and plaster of Paris jackets in tuberculosis of the spine in children on standard chemotherapy. Studies in Masan and Pusan. J Bone Joint Surg Br 1976;58-B:399–411.
A five years assessment of controlled trial of ambulatory treatment, debridement and anterior spinal fusion in the management of tuberculosis of the spine. J Bone Joint Surg Br 1978;60-B:163–77.
A controlled trial of anterior spinal fusion and debridement in the surgical management of tuberculosis of the spine in patient on standard chemotherapy. A study in two centres in South Africa. Tubercle 1978;59:79–105.
10 years assessement of anterior spinal fusion and debridement in the surgical management of tuberculosis of the spine in patient on standard chemotherapy in Hong Kong. J Bone Joint Surg Br 1982;64:393–8.
A 10 years assessment of controlled trial of inpatient and outpatient treatment and plaster of Paris jackets in tuberculosis of the spine in children on standard chemotherapy. J Bone Joint Surg Br 1985;67B:103–10.
A controlled trial of 6 months and 9 months regimens of chemotherapy in patients undergoing radical surgery for TB spine in Hong Kong. Tubercle 1986;67:243–59.
Controlled trial of short course regimen of chemotherapy in the ambulatory treatment of spinal tuberculosis. Results at 3 years of study in Korea. J Bone Joint surg Br 1993;75:240–8.
15 year assessment of controlled trials of short course chemotherapy regimens of 6, 9 and 18 months duration for spinal tuberculosis in patients ambulatory from the start of undergoing radical surgery. Int J Orthop 1999;23:73–81.
A controlled trial of short course regimen of chemotherapy in patients receiving ambulatory treatment or undergoing radical surgery for tuberculosis of the spine. Indian J Tuberc 1989;36 (supp):1–21.
Moon MS, Moon YW, Moon JL, Kim SS, Sun DH. Conservative treatment of tuberculosis of the lumbar and lumbo sacral spine. Clin Orthop Relat Res 2002;398:40–9.
Ramachandran S, Clifton IJ, Collyns TA, Watson JP, Pearson SB. The treatment of spinal tuberculosis: a retrospective study. Int J Tuberc Lung Dis 2005;9:541–44.
Upadhyay SS, Saji MJ, Yau AC. Duration of antitubercular chemotherapy in conjunction with radical surgery in the management of spinal tuberculosis. Spine 1996;21:1898–903.
Nene A, Bhojraj S. Results of non surgical treatment of thoracic spinal tuberculosis in adults. Spine J 2005;5:79–84.
Tuli SM. Tuberculosis of the skeletal system. 4th ed. New Delhi: Jaypee Brothers; 2004. p.3–5, 25–41, 200–27, 239–99.
Jain AK, Sinha S. Evaluation of system of grading of neurological deficit in tuberculosis of spine. Spinal Cord 2005;43:375–80.
Bhan S, Nag HL. Skeletal Tuberculosis. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2009.
Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br 1994;76:863–9.
Sharif HS, Clark DC, Aabed MY, Haddad MC, al Deeb SM, Yaqub B, et al. Granulomatous spinal infections: MR imaging. Radiology 1990;177:101–7.
Andronikou S, Jadwat S, Douis H. Patterns of disease on MRI in 53 children with tuberculous spondylitis and the role of gadolinium. Pediatr Radiol 2002;32:798–805.
Jain AK, Sreenivasan R, Saini NS, Kumar S, Jain S, Dhammi IK. Magnetic resonance evaluation of tubercular lesion in spine. Int Orthop 2012;36:261–9.
Smith AS, Weinstein MA, Mizushima A, Coughlin B, Hayden SP, Lakin MM, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol 1989;153:399–405.
Lee IS, Lee JS, Kim SJ, Jun S, Suh KT. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography imaging in pyogenic and tuberculous spondylitis: Preliminary study. J Comput Assist Tomogr 2009;33:587–92.
Hoffman EB, Crosier JH, Cremin BJ. Imaging in children with spinal tuberculosis. A comparison of radiography, computed tomography and magnetic resonance imaging. J Bone Joint Surg Br 1993;75:233–9.
Gillams AR, Chaddha B, Carter AP. MR appearance of temporal evolution and resolution of infectious spondylitis. Am J Roentgenol 1996;166:903–7.
Cormican L, Hammal R, Messenger J, Milburn HJ. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J 2006;82:46–51.
Schmitz A, Kälicke T, Willkomm P, Grünwald F, Kandyba J, Schmitt O. Use of fluorine-18 fluoro-2-deoxy-D-glucose positron emission tomography in assessing the process of tuberculous spondylitis. J Spinal Disord 2000;13:541–4.
Jain AK, Dhammi IK, Modi P, Kumar J, Sreenivasan R, Saini NS. Tuberculosis spine: Therapeutically refractory disease. Indian J of Orthop, 2012;46:171–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, A.K., Srivastava, A., Saini, N.S. et al. Efficacy of extended DOTS category I chemotherapy in spinal tuberculosis based on MRI-based healed status. IJOO 46, 633–639 (2012). https://doi.org/10.4103/0019-5413.104191
Published:
Issue Date:
DOI: https://doi.org/10.4103/0019-5413.104191