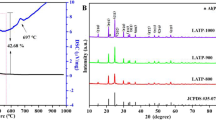
Abstract
Li0.33La0.56TiO3 (LLTO) solid state electrolytes have been considered as candidates to substitute for organic liquid electrolytes in lithium batteries. LLTO consist of a mixture of cubic phases with Pm3m symmetry (α-LLTO) and with tetragonal P4/mmm symmetry (β-LLTO). The α-LLTO phase has a higher Li ion conductivity than the β-LLTO phase. However, α-LLTO is characterized by high lattice strain, which induces the formation of β-LLTO due to the different atomic sizes of Li+ (0.76 Å) and La3+ (1.03 Å). In this study, to reduce lattice stress, we prepared Y-doped Li0.33La0.56−xYxTiO3 (x = 0.02, 0.05, 0.1) by using the sol-gel method. Because the ionic radius of Y3+ is 0.90 Å, that is, larger than that of Li+ (0.76 Å) and smaller than that of La3+ (1.03 Å), Y3+ suppresses the lattice distortion that prevents the formation of α-LLTO. The structural, morphological, and electrical characteristics of pure and Y3+-doped LLTO were investigated. The results show that Li0.33La0.46Y0.1TiO3 exhibits a better bulk conductivity (σ = 9.51 × 10−4 S cm−1) at 25 °C due to the increasing volume fraction of the more conductive α-LLTO phase.
Similar content being viewed by others
References
P. Knauth, Solid State Ion. 180, 911 (2009).
J. W. Fergus, J. Power Sources 195, 939 (2010).
L. Wüllen, T. Echelmeyer, H. W. Meyer and D. Wilmer, Phys. Chem. Chem. Phys. 9, 3298 (2007).
E. J. Cussen, J. Mater. Chem. 20, 5167 (2010).
S. Narayanan, F. Ramezanipour and V. Thangadurai, J. Phys. Chem. C 116, 20154 (2012).
V. Thangadurai and W. Weppner, J. Am. Ceram. Soc. 88, 411 (2005).
R. Kanno and M. Murayama, J. Electrochem. Soc. 148, A742 (2001).
A. D. Robertson, S. G. Martin, A. Coats and A. R.West, J. Mater. Chem. 5, 1405 (1995).
A. Várez, F. G. Alvarado, E. Morán and M. A. A. Franco, J. Solid State Chem. 118, 78 (1995).
T. Teranishi, A. Kouchi, H. Hayashi and A. Kishimoto, Solid State Ion. 263, 33 (2014).
Y. Harada, T. Ishigaki, H. Kawai and J. Kuwano, Solid State Ion. 108, 407 (1998).
Y. Harada, Y. Hirakoso, H. Kawai and J. Kuwano, Solid State Ion. 121, 245 (1999).
T. Teranishi, M. Yamamoto, H. Hayashi and A. Kishimoto, Solid State Ion. 243, 18 (2013).
H. T. T. Le, D. T. Ngo, Y-J. Kim, C-N. Park and C. J. Park, Electrochimica Acta 248, 232 (2017).
H. Zhang, S. Hao and J. Lin, Alloys Compd. 704, 109 (2017).
A. Mei, X-L. Wang, J-L. Lan, Y-C. Feng, H-X. Geng, Y-H. Lin and C-W. Nan, Electrochimica Acta 55, 2958 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SJ., Bae, JJ. & Son, JT. Structural and Electrical Effects of Y-doped Li0.33La0.56−xYxTiO3 Solid Electrolytes on All-Solid-State Lithium Ion Batteries. J. Korean Phys. Soc. 74, 73–77 (2019). https://doi.org/10.3938/jkps.74.73
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3938/jkps.74.73