Abstract
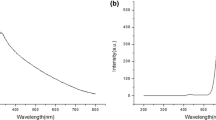
In this study, water-dispersible manganese (II)-ion-doped ZnS nanocrystals (ZnS:Mn NCs) were synthesized by capping the surface with polar cysteamine (Cyst) molecules. The obtained NC products were physically and optically characterized using various spectroscopic methods. Additionally, the surface charge and the degree of aggregation for the ZnS:Mn-Cyst NCs in water were determined using electrophoretic and hydrodynamic light scattering methods. The results indicated the formation in water of NC agglomerates with an average size of 278 nm and a positive surface charge (+10.39 mV) at ambient temperature. The positively charged NCs were applied as photosensors for the detection of a specific anion in an aqueous solution. As a result, the ZnS:Mn-Cyst NCs showed an exclusive luminescence quenching effect upon addition of nitrite (NO2− ) ions. Finally, the Stern-Volmer kinetic study on the luminescence quenching mechanism proposed an energy transfer via dynamic collisions between the oppositely charged ZnS:Mn-Cyst NCs and NO2− ions.
Similar content being viewed by others
References
L. Xu, X. Qi, X. Li, Y. Bai and H. Liu, Talanta 146, 714 (2016).
C. R. Kagan and C. B. Murray, Nature Nanotech. 10, 1013 (2015).
C. Delerue, Nature Mater. (2016), [DOI: 10.1038/nmat4597].
L. Lin, M. Zapata, M. Xiong, Z. Liu, A. Wang, H. Xu, A. G. Borisov, H. Gu, P. Nordlander, J. Aizpurua and J. Ye, Nano Lett. 15, 6419 (2015).
N. K. Elumalai, C. Vijila, R. Jose, A. Uddin and S. Ramakrishina, Mater. Renew. Sustain. Energy 4, 11 (2015).
S. A. Bhakta, E. Evans, T. E. Beavidez and D. Garcia, Anal. Chim. Acta 872, 7 (2015).
W. W. Yu, Biochem. Biophys. Res. Commun. 348, 781 (2006).
R. C. Somers, M. G. Bawendi and D. G. Nocera, Chem. Soc. Rev. 36, 579 (2007).
V. Brunetti, H. Chibli, R. Fiammengo, A. Galeone, M. A. Malvindi, G. Vecchio, R. Cingolani, J. L. Nadeau and P. P. Pompa, Nanoscale 5, 307 (2013).
W. W. Yu, E. Chang, R. Drezek and V. L. Colvin, Biochem. Biophys. Res. Commun. 346, 781 (2006).
L. Li, Y. Cheng, Y. Ding, Y. Lu and F. Zhang, Anal. Meth. 4, 4213 (2012).
K. Chen, H. Shen, Z. Wang, A. Zhou and Q. Xia, Am. J. Anal. Chem. 6, 1 (2015).
J. H. Lee, Y. A. Kim, K. M. Kim, Y. D. Huh, J. W. Hyun, H. S. Kim, S. J. Noh and C. S. Hwang, Bull. Korean Chem. Soc. 28, 1091 (2007).
S. Park, B. Song, H. Y. Kong, J. Byun and C. S. Hwang, Bull. Korean Chem. Soc. 35, 1169 (2014).
D. Vasudevan, A. Trinchi, S. G. Hardin and I. S. Cole, J. Lumin. 166, 88 (2015).
M. B. Gumpu, S. Sethuraman, U. M. Krishnan, J. Bosco and B. Rayappan, Sensors Actuators B 213, 515 (2015).
J. Heo and C. S. Hwang, Bull. Korean Chem. Soc. 36, 2411 (2015).
C. S. Hwang, N. Lee, Y. A. Kim and Y. B. Park, Bull. Korean Chem. Soc. 27, 1809 (2006).
R. N. Bhargava, D. Gallagher, X. Hong and A. Nurmikko, Phys. Rev. Lett. 72, 416 (1994).
T. Kushida, Y. Tanaka and Y. Oka, Solid. State Commun. 14, 617 (1974).
I. Yu, T. Isobe and M. Senna, J. Phys. Chem. Solids 57, 373 (1996).
N. Karar, F. Singh and B. R. Mehra, J. Appl. Phys. 95, 656 (2004).
A. T. R. Williams, S. A. Winfield and J. N. Miller, Analyst 108, 1067 (1983).
S. R. Meech and D. Philips, J. Photochem. 23, 193 (1983).
J. H. Lee, Y. A. Kim, K. M. Kim, Y. D. Huh, J. W. Hyun, H. S. Kim, S. J. Noh and C. S. Hwang, Bull. Korean Chem. Soc. 28, 1091 (2007).
International Union of Crystallography in International Tables for X-ray Crystallography, Part III (Dordrecht, Netherlands, 1985).
F. W. Jones, in Proceedings of Royal Society of London. Series (The Royal Society Publishing, Terrace, London, UK, 1937).
L. J. Willis, T. M. Loehr, K. F. Miller, A. E. Bruce and E. I. Stiefl, Inorg. Chem. 25, 4289 (1986).
L. Riauba, Chemija 18, 1 (2007).
K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds (John Wiley & Son, New York, USA, 1997).
A. Kudelski and W. Hill, Langmuir 15, 3162 (1999).
C. W. Moszczenski and R. J. Hooper, Inorg. Chim. Acta 70, 71 (1983).
M. Ivanda et al., J. Raman Spectrosc. 38, 647 (2007).
C. Urban and P. Schurtenberger, J. Colloid Interface Sci. 207, 150 (1988).
S. Park, B. Song, H. Y. Kong, J. Byun and C. S. Hwang, Bull. Korean Chem. Soc. 35, 1169 (2014).
J. Josefwicz and F. R. Hallett, Appl. Opt. 14, 740 (1975).
X. Liu, L. Guo, L. Cheng and H. Ju, Talanta 78, 691 (2009).
P. Wu, T. Zhao, S. Wang and X. Hou, Nanoscale 6, 43 (2014).
H. Liu, G. Yang, E. S. Abdel-Halim and J. J. Zhu, Talanta 104, 135 (2013).
D. M. Hirst and J. W. Linnet, J. Chem. Phys. 43, 574 (1965).
B. Unnikrishnan, S. C. Wei, W. J. Chiu, J. Cang, P. H. Hsu and C. C. Huang, Analyst 139, 2221 (2014).
A. Ianoul, T. Coleman and S. A. Asher, Anal. Chem. 74, 1458 (2002).
I. E. Borissevitch, J. Lumin. 81, 219 (1999).
A. Alonso, B. Etxaniz and M. D. Martinez, Food Add. Cont. 9, 111 (1992).
K. K. Choi and K. W. Fung, Analyst 105, 241 (1980).
S. Q. Wang, Y. M. Yin and X. Q. Lin, Electrochem. Commun. 6, 259 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sim, Y., Hwang, CS. Photosensor Activities of Cysteamine-Capped ZnS:Mn Nanocrystals in the Direct Detection of Nitrite Ions by Fluorescence Quenching in Aqueous Solutions. Journal of the Korean Physical Society 72, 424–430 (2018). https://doi.org/10.3938/jkps.72.424
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3938/jkps.72.424