Abstract
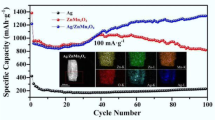
The present work investigates the electrochemical response of silver nanoparticle (Ag-NP)-decorated TiO2 nanotube (NT) layers as an anode material for a lithium-ion battery. Self-organized nanotube layers with a thickness of approximately 1 µm and a diameter of approximately 100 nm were grown by anodization of Ti in a fluoride-containing aqueous electrolyte. Ag NPs (average particle size of ∼10 nm) were deposited both inside and outside the nanotube geometry in a well-distributed manner through a simple and efficient photocatalytic reduction process. The morphology and the chemical composition of the resulting materials were characterized by using field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD) and energy dispersive X-ray spectroscopy (EDX). Our results show that the TiO2 NT layers decorated with Ag NPs had a superior electrochemical response in terms of charge/discharge capacity, rate capability, cyclic performance and columbic efficiency. The enhanced performance is attributed to the improved electronic and ionic conductivity, obtained by providing highly conductive paths to electrons flowing through a well-distributed Ag NPs deposition on the walls of the highly-oriented NTs.
Similar content being viewed by others
References
T. D. Tran, J. H. Feikert, X. Song and K. Kinoshita, J. Electrochem. Soc. 142, 3297 (1995).
D. Aurbach, Y. Ein-Eli, O. Chusid, Y. Carmeli, M. Babai and H. Yamin, J. Electrochem. Soc. 3, 603 (1994).
M. Winter and J. O. Besenhard, Electrochim. Acta 45, 31 (1999).
M. Wagenaker, A. P. M. Kentgens and F. M. Mulder, Nature 418, 397 (2002).
P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem. Int. Ed. 47, 2930 (2008).
A. N. Jansen, A. J. Kahaian, K. D. Kepler, P. A. Nelson, K. Amine, D. W. Dees, D. R. Vissers and M. M. Thackeray, J. Power Sources 5, 902 (1999).
A. R. Armstrong, G. Armstrong, J. Canales and P. G. Bruce, J. Power Sources 146, 501 (2005).
M. A. Reddy, M. S. Kishore, V. Pralong, V. Caignaert, U. V. Varadaraju and B. Raveu, Electrochemistry Commun. 8, 1299 (2006).
G. F. Ortiza, I. Hanzua, P. Knauth, P. Lavela, J. L. Tiradob and T. Djenizian, Electrochem. Acta 54, 4262 (2009).
X. P. Gao, Y. Lan, H. Y. Zhu, J. W. Liu, Y. P. Ge, F. Wu and D. Y. Song, Electrochem. Solid-State Lett. 8, A26 (2005).
C. H. Jiang, I. Honma, T. Kudo and H. S. Zhou, Electrochem. Solid-State Lett. 10, A127 (2007).
C. H. Jiang, I. Honma, T. Kudo and H. S. Zhou, J. Power Sources 166, 239 (2007).
A. R. Armstrong, G. Armstrong, J. Canales, R. García and P. G. Bruce, Adv. Mater. 17, 862 (2005).
Y. G. Guo, Y. S. Hu, W. Sigle and J. Maier, Adv. Mater. 19, 2087 (2007).
Z. Ali, S. N. Cha, J. I. Sohn, I. Shakir, C. Yan, J. M. Kim and D. Kang, J. Mater. Chem. 22, 17625 (2012).
G. F. Ortiz, I. Hanzu, T. Djenizian, P. Lavela, J. L. Tirado and P. Knauth, Chem. Mater. 21, 63 (2009).
N. A. Kyeremateng, V. Hornebecq, P. Knauth and T. Djenizian, Electrochimia Acta 62, 192 (2012).
N. A. Kyeremateng, F. Vacandio, M. T. Sougrati, H. Martinez, J. C. Jumas, P. Knauth and T. Djenizian, J. Power Sources 224, 269 (2013).
G. F. Ortiz, I. Hanzu, P. Knauth, P. Lavela, J. L. Tirado and T. Djenizian, Electrochem. Solid-State Lett. 12, A186 (2009).
I. Paramasivam, J. M. Macak, A. Ghicov and P. Schmuki, Chemical Physics Lett. 445, 233 (2007).
D. B. Ingram and S. Linic, J. American Chem. Soc. 133, 5202 (2011).
I. Paramasivam, J. M. Macak, A. Ghicov and P. Schmuki Electrochemistry Comm. 10, 71 (2008).
J. M. Macak and P. Schmuki, Electrochem. Acta 52, 1258 (2006).
K Chen, X. Feng, R. Hu, Y. Li, K. Xie and H. Gu, J. Alloys Comp. 554, 72 (2013)
B. Zhonghe, M. P. Paranthaman, B. Guo, X. G. Sun and S. Dai, J. Power Sources 222, 461 (2013).
W. H. Ryu, D. H. Nam, Y. S. Ko, R. H. Kim and H. S. Kwon, Electrochem. Acta 61, 19 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pervez, S.A., Farooq, U., Yaqub, A. et al. Improved performance of Ag-nanoparticle-decorated TiO2 nanotube arrays in Li-ion batteries. Journal of the Korean Physical Society 63, 1809–1814 (2013). https://doi.org/10.3938/jkps.63.1809
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3938/jkps.63.1809