Abstract
David Sherry has been a pioneer in investigating the avian hippocampal formation (HF) and spatial memory. Following on his work and observations that HF is sensitive to the occurrence of reward (food), we were interested in carrying out an exploratory study to investigate possible HF involvement in the representation goal value and risk. Control sham-lesioned and hippocampal-lesioned pigeons were trained in an open field to locate one food bowl containing a constant two food pellets on all trials, and two variable bowls with one containing five pellets on 75% (High Variable) and another on 25% (Low Variable) of their respective trials (High-Variable and Low-Variable bowls were never presented together). One pairing of pigeons learned bowl locations (space); another bowl colors (feature). Trained to color, hippocampal-lesioned pigeons performed as rational agents in their bowl choices and were indistinguishable from the control pigeons, a result consistent with HF regarded as unimportant for non-spatial memory. By contrast, when trained to location, hippocampal-lesioned pigeons differed from the control pigeons. They made more first-choice errors to bowls that never contained food, consistent with a role of HF in spatial memory. Intriguingly, the hippocampal-lesioned pigeons also made fewer first choices to both variable bowls, suggesting that hippocampal lesions resulted in the pigeons becoming more risk averse. Acknowledging that the results are preliminary and further research is needed, the data nonetheless support the general hypothesis that HF-dependent memory representations of space capture properties of reward value and risk, properties that contribute to decision making when confronted with a choice.
Similar content being viewed by others
Introduction
In his seminal studies investigating the memory systems of food-storing songbirds (Sherry et al., 1989; Sherry & Vaccarino, 1989), David Sherry established the avian hippocampal formation (HF) as an important comparative model for understanding the relationship between the hippocampus and memory processes. The one general message following from Sherry’s foundational research is the critical role the avian HF plays in supporting spatial memory (Colombo & Broadbent, 2000; Herold et al., 2015; Pravosudov & Smulders, 2010; Sherry et al., 1989, 1992; Smulders, 2006). Specifically, HF lesions in several food-storing species revealed memory deficits in relation to spatial, but not object centered, goal information (Clayton & Krebs, 1995; Hampton & Shettleworth, 1996; Shiflett et al., 2003). Additionally, evidence from immediate early gene (IEG) studies further demonstrates the overall importance of the avian HF for space-based memory in both food-storing and non-storing avian species (Bischof et al., 2006; Coppola & Bingman, 2020; Mayer & Bischof, 2012; Mayer et al., 2016; Mayer et al., 2018; Mayer et al., 2010; Smulders & DeVoogd, 2000).
In homing pigeons, HF research has principally focused on the relationship between the HF and navigation (see Herold et al., 2015, for a review), but there is a substantial body of literature examining the relationship between HF and spatial memory for food locations (Bingman et al., 2006; Bingman & Jones, 1994; Colombo & Broadbent, 2000; Johnston et al., 2021; Nardi & Bingman, 2007; Strasser & Bingman, 1997, 1999; Vargas et al., 2004). Even in a species that does not cache food, what is interesting about the relationship between the homing pigeon HF and spatial memory for food locations is that it implies some modulation of HF-dependent spatial representations by the experience of reward. Indeed, in the mammalian hippocampus literature, there is a growing recognition that hippocampal-dependent spatial maps are influenced by the spatial distribution and properties of reward occurrence (Jeong et al., 2018; Jin & Lee, 2021; Jung et al., 2018; Mamad et al., 2017; Mizumori & Tyron, 2015; Mizumori et al., 2009; Mizumori et al., 2004; Penner & Mizumori, 2012; Retailleau et al., 2012; Tryon et al., 2017; Wiener, 1993; Wood et al., 2000; see Sosa & Giocomo, 2021, for a review). Similarly, avian HF-dependent spatial representations also appear strongly influenced by how rewards are distributed in space. Homing pigeon HF neurons display a disproportionate number of increased firing fields at reward locations (Hough & Bingman 2004; Siegel et al., 2005; contrast with Kahn et al., 2008). Although offering only indirect support, it is also notable that the spatial response properties of HF neurons in a food-caching songbird species are more robust than in a comparison, non-storing species (Payne et al., 2021). Following from the electrophysiological data, HF lesions disrupt the ability of homing pigeons to uniquely encode the spatial location of a desirable compared to a less desirable food reward (Kahn & Bingman, 2009), while similar HF lesions have no effect on discriminating a desirable from a less desirable reward based on feature discriminative stimuli (Coppola et al., 2014).
The current study builds from the above by broadening the inquiry into the relationship among the avian HF, discriminative stimuli, and reward properties by carrying out an exploratory investigation of whether HF lesions influence homing pigeon memory for reward probability or risk. Pigeons are well documented to discriminate cues based on reinforcement probabilities during goal-seeking behavior (Bullock & Bitterman, 1962; Graf et al., 1964; Roberts et al., 2015; Roberts et al., 2018; Scarf et al., 2014) and pigeons with HF lesions display impaired performance in a differential reinforcement probability task (Scarf et al., 2014). Of particular interest for the current study is the question of whether HF lesions would influence decision making related to how pigeons represent memories for a modest constant (continual reinforcement) reward (food) compared to a riskier, variable reward (variable reinforcement schedule) of greater gain, which in the long run would yield a greater or lesser net food gain. Further, might any HF lesion effect be modulated by whether the discriminative stimuli capturing the different outcomes are spatial/location based or feature/object based? As further background, it should be noted that pigeons are not easily classified as risk prone or risk averse as they have been reported to be risk prone, risk averse, or indifferent to risk depending on the testing protocol (Essock & Reese, 1974; Hamm & Shettleworth, 1987; Lagorio & Hackenberg, 2012; Ludvig et al., 2014; Menlove et al., 1979; Smith et al., 2017; Staddon & Innis, 1966; Young, 1981). Following from the observations that the hippocampal spatial memory system is responsive to the occurrence of reward, we predicted an admittedly unspecified effect of HF lesions on how homing pigeons choose among goal locations that vary with respect to reward value and risk, and that any effect would be most pronounced when memory for the distribution of reward and associated risk is encoded based on goal location/space.
Method
Feature Task: Subjects
Sixteen unsexed adult homing pigeons (Columba livia) were obtained from local racing hobbyists. One pigeon died due to natural causes unrelated to the experiment before testing began. Pigeons were randomly assigned to one of two experimental conditions: HF-lesion group (HF, n = 7) or sham-lesioned control group (C, n = 8). Birds were individually housed in metal cages (56 × 38 × 31 cm) in a temperature and humidity-controlled colony-housing room with a 14/10 light-dark cycle. Lights were turned on at 8:00 local time and turned off at 22:00 local time. Birds were food restricted to no less than 80% of their free-feeding weights, had access to food during training, and were supplementally fed in their home cages to maintain weight. Water was provided ad libitum. All procedures were approved by the Bowling Green State University Institutional Animal Care and Use Committee.
Feature Task: Hippocampal formation electrolytic lesion surgery
Homing pigeons were food deprived 18–24 h prior to surgery. Anesthesia was induced in a small plastic chamber using isoflurane gas. Animals remained in the induction chamber until the anesthetic took full effect, as evidenced by a lack of response to a toe pinch, shallow breathing, and depression of pulse and blood pressure. Once anesthetized, the feathers covering the ear openings and the top of the head were clipped. Pigeons were transferred to a stereotaxic apparatus with an attached nose cone that continued to deliver gas anesthetic throughout the surgery. The skin covering the skull was cut with a scalpel and held open with hemostats. Using stereotaxic methods, six target coordinates for bilateral hippocampal lesions were located. A portion of the skull was removed with a high-speed drill, and an electrode (stainless steel pin insulated with Epoxylite) was inserted horizontally (parallel to the anterior-posterior axis of a pigeon’s head) into the brain. The eight sham-lesioned control animals underwent the same procedure, except they did not have electrodes inserted into their brains.
For the seven bilateral HF-lesioned pigeons, the target lesion coordinates for each brain hemisphere (Lesion 1 = A 3.8, L ± 0.3, V 12.2; Lesion 2 = A 3.8, L ± 0.5, V 13.3; Lesion 3 = A 3.5, L ± 1.0, just ventral to the surface of the brain) were determined according to the pigeon atlas of Karten and Hodos (1967). At each of these locations, 3.0 μA of current was applied for 15 s (Lesion 1) or 20 s (Lesions 2 and 3), using a 5-mm exposed electrode tip. Following the lesions, electrodes were removed from the brain, and the skin over the skull was closed with sterile wound clips. All pigeons were placed in a recovery chamber until they regained consciousness, at which time they returned to their original cage. After between 1 and 2 weeks of recovery time, the wound clips were removed from all animals, and behavioral training began.
Feature Task: Stimuli and materials
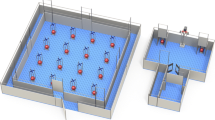
The experimental room was rich in 2-D visual cues attached to the walls and 3-D landmark objects and its dimensions were 3.38 m × 4.28 m with eight fluorescent lights mounted on the ceiling (Fig. 1). In the Feature Task, five colored bowls (e.g., green, red, yellow, blue, brown) were positioned in a centered, linear array approximately 100 cm from two parallel walls. The linear array setup could be horizontal (as depicted in Fig. 1a) or vertical with 32 cm between each bowl. There were two release sites per array orientation, four in total (N-S-E-W walls). Colored bowls were pseudo-randomly shifted to various locations within the linear arrays of either orientation (horizontal or vertical) between trials and sessions, with the constraint that the same-colored bowl could not be placed at the same location within the linear array more than three times per session (eight trials/session). The location of the bowls changed across trials or sessions depending on the orientation of the linear array deployed, and randomly assigned candidate food reward sites were always associated with the same three colored bowls (e.g., blue, red, green), although the color of a bowl at each site constantly changed. Again, only four bowls were available during each trial (see below). Birds were pseudo-randomly released from each of the four release sites twice per session; relative to the observer, E/W wall release sites were used during horizontal array orientations, and N/S release sites were used during vertical array orientations.
a Feature Task Environment (horizontal alignment depicted). Two release sites (X) were used. Five bowls (B, blue; Y, yellow; G, green; BR, brown; R, red) were positioned in a horizontal alignment in the center of the room. The horizontal alignment position of any given bowl changed across sessions (vertical alignment not shown). Different probabilities of reward reinforcement were associated with specific colors: Constant – 100% (2 pellets × 4 trials per each trial type = 8 pellets available; combined total of 16 pellets available per session overall: 2 pellets × 8 trials), 0% (zero pellets), 0% (zero pellets), Low Variable – 25% (5 pellets × 1 trial = 5 pellets available), High Variable – 75% (5 pellets × 3 trials = 15 pellets available). b Space Task Environment. Four release sites (X) were used. Five different colored (blue, green, red, brown, yellow) bowls were positioned at distinct spatial locations (1, 2, 3, 4, 5). Bowls changed position across sessions. Different probabilities of reward reinforcement were associated with specific locations, similar to the Feature Task (100%, 0%, 0%, 25%, 75%). Two-dimensional wall posters (P), various three-dimensional objects (broom; T, artificial tree; BL, black cone; G, green garbage can), three wooden triangles (LG, light green; Y, yellow; W, white), and two striped boards (black and white) provided a robust visuospatial environment
Feature Task: Pretraining
All birds were trained in the same room for both pretraining and the behavioral experimental tasks. During pretraining, birds were gradually introduced to the empty (no 2-D visual cues or landmark objects) experimental environment over the course of several days. At this stage, birds were randomly placed on the floor in different locations in the room with four white bowls filled with grit; the position of the bowls on the floor changed daily. Initially, the room’s lights were turned off, but once a bird was on the floor of the room and the researcher was behind the entrance door, the lights were turned on for the entire duration of a pretraining trial. The lights were turned off again at the end of the trial. In the beginning, food was scattered across the floor and placed on the top of each bowl. Once a bird became more familiar with the room and ate all the food off the floor, food availability was gradually reduced until all food was buried under grit in each bowl. Pretraining trials continued until a bird found buried food in all four bowls within 5 min.
Feature Task: Procedure
Pigeons were trained 6 days per week and sessions consisted of eight trials (one session/day). On any trial, two bowls could have contained food, and pigeons were presented with two different types of trials: four High Variable and four Low Variable. For both trial types, there was a specific-colored bowl (e.g., blue) designated as the constant reward color baited with food (two pellets) for each pigeon. For every trial (four of each different type per session), this same rewarded color bowl contained food, resulting in eight possible pellets (2 pellets × 4 trials) per session for each trial type (combined total of 16 pellets per eight trials/session: 2 pellets × 8 trials overall).
On High-Variable trials, during 75% of the four trials/session (three of four trials), the colored bowl (e.g., red) designated as High-Variable color was baited with five pellets; for the remaining trial, no food was present in the bowl (zero pellets). If during the four High-Variable trials of a session a pigeon preferentially chose the High-Variable bowl, they would obtain 15 total pellets (5 pellets × 3 trials), exceeding the return from the Constant bowl by seven pellets (15 − 8 = 7). By contrast, during Low-Variable trials, on 25% of the four trials/session (one of four trials), the colored bowl (e.g., green) designated as Low-Variable color was baited with five pellets; for the three remaining trials, no food was present in the bowl (zero pellets). If during the four Low-Variable trials of a session a pigeon preferentially chose the Low-Variable bowl, they would obtain five total pellets (5 pellets × 1 trial), a net loss of three pellets compared to the Constant bowl across the four trials (5 − 8 = −3). Importantly, on High-Variable trials, the Low-Variable bowl from the Low-Variable trials was not present, and on Low-Variable trials, the High-Variable bowl from the High-Variable trials was not present (that is why only four bowls were present on any given trial, which included two bowls of never baited colors (e.g., brown, yellow).
In order to reduce the possible use of path integration to locate a goal (more important for the Space Task, see below), Feature pigeons were slowly rotated (approximately 10–12 rotations per min) while in a covered pet carrier on a swivel chair in a room adjacent to the experimental room for 1 min before the start of every trial (see Coppola et al., 2014; Kahn & Bingman, 2009; Vargas et al., 2004). Each trial began inside the darkened experimental room as a pigeon was pseudo randomly released from one of the four possible release positions with the constraint that each release position could not be used more than twice per session. Once a pigeon was placed at the release position on the floor of the experimental room, the researcher shut the room’s door, turned on the lights, and recorded a pigeon’s food bowl choices through a one-way glass window in the door. A pigeon was allowed to search for food for 5 min until it pecked into both potentially correct/rewarded colored bowls; the Constant bowl that was always baited (e.g., blue) and the possibly baited High-Variable (e.g., red) or Low-Variable (e.g., green) bowls.
A choice was defined as a peck into a bowl, and a pigeon had to move away from a bowl, defined as walking approximately 15 cm away from the bowl in any direction, and return in order for a second choice to be recorded for the same bowl. Bowl choices and latencies were recorded. However, for the current paper, we are only reporting the first-choice data, which were the most revealing. Once a pigeon pecked into both candidate baited/correct bowls or did not choose at all after 5 min, the lights were turned off, and the pigeon was removed from the room. If a pigeon did not choose a bowl after 5 min, the trial was terminated. Terminated trials were repeated until a pigeon completed the trial. There was a 3-min inter-trial interval in which a pigeon was placed in a covered pet carrier in the room adjacent to the experimental room. During this interval, the researcher cleaned the experimental room’s floor of debris, changed the arrangement of the colored bowls, and rebaited the appropriate bowls. Pigeons in the Feature Task were trained for 16 sessions.
Feature Task: Histology and lesion reconstruction
After completion of the experimental study, the seven HF-lesioned pigeons were injected with a lethal dose of sodium pentobarbital (100 mg/kg intramuscularly) and perfused intracardially with 0.9% saline followed by a fixative solution (10% formalin). Brains were harvested and placed in 10% formalin for 24 h. The brains were embedded in a 30% sucrose solution in phosphate buffer saline (PBS) for 48–96 h for cryoprotection. They were sliced into 50 μm thick sections on a freezing microtome. Every fourth section was mounted on a gel-coated slide, stained with Cresyl violet, and coverslipped. The extent of the lesion damage was examined using a Micromaster Fisher Scientific microscope. Lesions were reconstructed on standard coronal sections adapted from the pigeon atlas (Karten & Hodos, 1967).
Feature Task: Data analysis
In order to compare the two groups on the feature-based, risk-reward memory task, the total number of correct first choices across the training sessions were assessed using a 2 (group: Control, HF) × 4 (sessions 1–4, 5–8, 9–12, 13–16) mixed-model ANOVA. Analyses were performed using SPSS Statistics, Version 26.0 (IBM, Armonk, NY, USA). The criterion for statistical significance was p < 0.05.
Space Task: Subjects
By contrast to the expectation of no effect of HF lesions on the Feature Task, we expected to observe an effect of HF lesions on the Space Task when food goals were discriminated based on their locations in space. Test subjects were planned to be run for the same number of 16 sessions, and although the data suggested altered learning on the part of the HF-lesioned subjects after those 16 sessions (data not presented), we felt that additional training would show an even bigger effect. Therefore, pigeons were randomly assigned to one of two experimental conditions: a hippocampal-lesion group (HF, n = 5) or a sham-lesioned control group (C, n = 5) and were trained for 20 sessions. The housing and the maintenance of the pigeons were the same as the Feature Task.
Space Task: Hippocampal formation electrolytic lesion surgery
The surgery procedures were identical to those of Feature Task.
Space Task: Stimuli and materials
The Space Task’s experimental room floor dimensions were 3.38 m × 4.28 m with eight fluorescent lights mounted on the ceiling (same room used in Feature Task). The room was rich in 2-D visual cues attached to the walls and 3-D landmark objects. Observers stood outside of the room behind a door with a one-way mirror during shaping and training. For the Space Task, five bowls differing only in color, with approximately 135 cm between each bowl, were positioned at five distinct, constant spatial locations in the experimental room (Fig. 1b). Colored bowls were pseudo randomly shifted to any of the five locations between trials and sessions, with the constraint that the same-colored bowl could not be placed at any one location more than three times per session (eight trials/session, see below). The bowls' relative room location did not change across trials or sessions, and the three randomly assigned candidate food reward sites were baited at the same location and remained stable for each bird throughout training, although the bowl color (e.g., green, red, yellow, blue, brown) at each site regularly changed. Only four bowls were deployed during each trial (see below). Birds were pseudo-randomly released from each of the four release sites (dice roll: 1 – North wall, 2 – South wall, 3 – West wall, 4 – East wall) twice per session.
Space Task: Pretraining
The pretraining was identical to the pretraining of the Feature Task.
Space Task: Procedure
Pigeons were trained 6 days per week and sessions consisted of eight trials (one session/day). On any trial, there were two bowl locations that could have contained food, and pigeons were presented with two different types of trials: four High-Variable and four Low-Variable. For both trial types, there was a specific bowl location (e.g., Location 1) designated as the constant reward site baited with food (2 pellets × 4 trials) for each pigeon. For every trial (four of each of the two types per session), this same rewarded bowl location contained food, resulting in eight possible pellets per trial type (combined total of 16 pellets per eight trials/session: 2 pellets × 8 trials overall).
On High-Variable trials, during 75% of the four trials/session (three of four trials), the spatial location (e.g., Location 3) designated as High Variable was baited with five pellets; for the remaining trial, no food was present in the bowl at that location (zero pellets). If during the four High-Variable trials of a session a pigeon preferentially chose the High-Variable bowl, they would obtain 15 total pellets (5 pellets × 3 trials), exceeding the return from the Constant bowl by seven pellets (15 − 8 = 7). By contrast, during Low-Variable trials, on 25% of the four trials/session (one of four trials), the bowl’s spatial location (e.g., Location 5) designated as Low Variable was baited with five pellets; for the three remaining trials, no food was present in the bowl (zero pellets). If during the four Low-Variable trials of a session a pigeon preferentially chose the Low-Variable bowl’s spatial location, they would obtain five total pellets (5 pellets × 1 trial), a net loss of three pellets compared to the Constant bowl’s spatial location on all four trials (5 − 8 = −3). Importantly, on High-Variable trials, the Low-Variable spatial location from the Low-Variable trials was not present, and on Low-Variable trials, the High-Variable spatial location from the High-Variable trials was not present (that is why only four bowl sites were present on any given trial, which included two bowls of never baited spatial locations (e.g., Location 2, Location 4).
In order to reduce the possible use of path integration to locate a goal, which was especially important for the Space Task, pigeons were slowly rotated (approximately 10–12 rotations per min) while in a covered pet carrier on a swivel chair in a room adjacent to the experimental room for 1 min before the start of every trial (see Coppola et al., 2014; Kahn & Bingman, 2009; Vargas et al., 2004). Each trial began inside the darkened experimental room as a pigeon was pseudo-randomly released from one of the four possible release positions with the constraint that each release position could not be used more than twice per session. Once a pigeon was placed at the release position on the floor of the experimental room, the researcher shut the room’s door, turned on the lights, and recorded a pigeon’s food bowl choices through a one-way glass window in the door. A pigeon was allowed to search for food for 5 min until it pecked into both potentially correct bowls’ spatial locations; the Constant bowl that was always baited (e.g., Location 1) and the possibly baited High-Variable (e.g., Location 3) or Low-Variable (e.g., Location 5) bowl sites.
All other behavioral procedures were identical to the Feature Task.
Space Task: Histology and lesion reconstruction
The histology and hippocampal-lesion reconstruction procedures were identical to those of the Feature Task.
Space Task: Data analysis
In order to compare risk-reward space-based associative learning, the total number of correct choices across the training sessions were assessed using a 2 (group: Control, HF) × 5 (sessions 1–4, 5–8, 9–12, 13–16, 17–20) mixed-model ANOVA. Due to the exploratory nature of the Space Task, significance was set at p-values of < 0.10 and unpaired t-tests were employed to carry out post hoc tests. Analyses were performed using SPSS Statistics, Version 26.0 (IBM, Armonk, NY, USA).
Results
Feature Task: First choices to High-Variable and Constant bowls during High-Variable trials
Inspection of Fig. 2a reveals that both the C and HF pigeons were rational decision makers, similarly learning to preferentially choose first the High-Variable 75% bowl compared to the Constant 100% bowl. There was a main effect of blocked sessions F(3,39) = 6.74, p = 0.001, but not for group F(1, 13) = 2.20, p = 0.16. Additionally, there was not a significant interaction between blocked sessions and group F(3,39) = 0.75, p = 0.53. Also, across training, both the C and HF pigeons showed little change in the number of first choices to the Constant 100% bowl, with first choices to the Constant 100% bowl occurring on about 40% of the trials (Fig. 2b). There was no main effect of blocked sessions F(3,39) = 41.08, p = 0.34, nor group F(1,13) = 2.60, p = 0.13. Additionally, there was not a significant interaction between blocked sessions and group F(3,39) = 0.60, p = 0.62.
Learning progressions of the probability discrimination task by C and HF Feature birds. a Mean percent of first choices to High-Variable 75% color bowls during High-Variable sessions across four training blocks. b Mean percent of first choices to Constant 100% color bowls during High-Variable sessions across four training blocks. c Mean percent of first choices to Low-Variable 25% color bowls during Low-Variable sessions across four training blocks. d Mean percent of first choices to Constant 100% color bowls during Low-Variable sessions across four training blocks. Chance level was 25% for all graphs. Error bars indicate SEM. C control, HF hippocampal formation
Feature Task: First choices to Low-Variable and Constant bowls during Low-Variable trials
The High-Variable trials revealed no difference between the C and HF pigeons in their ability to learn to choose first the High-Variable 75% bowl when feature was used as the discriminative stimulus. A similar lack of difference was also found for the Low-Variable trials (Fig. 2c). There was a main effect of blocked sessions F(3,39) = 3.00, p = 0.04, reflecting fewer choices to the Low-Variable 25% bowl across training, but more importantly, there was no main effect for group F(1,13) = 0.37, p = 0.55. Additionally, there was not a significant interaction between blocked session and group F(3,39) = 1.30, p = 0.29. By contrast, both groups displayed an increase in the number of first choices to the Constant 100% bowl as training progressed (Fig. 2d). There was a main effect of blocked sessions F(3,39) = 11.08, p < 0.001, but not for group F(1,13) = 0.04, p = 0.85. Additionally, there was not a significant interaction between blocked sessions and group F(3,39) = 1.12, p = 0.35.
In summary, by preferring the High-Variable 75% bowl on High-Variable trials and the Constant 100% bowl on Low-Variable trials, both the C and HF pigeons performed as rational agents during the learning of the probability discrimination based on the color features of the goals. What is striking about the data is how similar the C and HF pigeons performed; there was no hint of a learning difference. The results are consistent with a similar study that did not include a risk/variable element in choice outcomes (Coppola et al., 2014), and is overall consistent with the literature (see Discussion), suggesting little effect of HF lesions when pigeons learn and remember feature properties to represent a goal.
Space Task: First choices to High-Variable and Constant locations during High-Variable trials
Visual inspection of Fig. 3a suggests that, as expected, HF lesions impaired the learning of directing first choices to the High-Variable 75% bowl and some of the statistical contrasts supported this visual impression. Reflecting the overall poorer performance of C pigeons on the Space Task, there was no main effect of blocked sessions F(4,32) = 1.70, p = 0.17. Importantly, there was an effect for group F(1, 8) = 3.38, p = 0.09. Additionally, there was not a significant interaction between blocked sessions and group F(4,32) = 1.11, p = 0.37. A post hoc analysis indicated that at the end training the C pigeons significantly outperformed (more High-Variable 75% first choices) the HF pigeons (BLOCK 5, C M = 53.75, SEM = 5.45; HF M = 23.75, SEM = 8.00; t(8) = 3.10, p = 0.02). By contrast, no group effect could be detected with respect to first choices to the Constant 100% bowl (Fig. 3b). There was an effect of blocked sessions as there seemed to be a modest increase in Constant 100% bowl first choices across training F(4,32) = 2.43, p = 0.07, but not for group F(1, 8) = 0.007, p = 0.94. Additionally, there was not a significant interaction between blocked sessions and group F(4,32) = 0.36, p = 0.83.
Learning progressions of the probability discrimination task by C and HF Space birds. a Mean percent of first choices to High-Variable 75% bowl locations during High-Variable sessions across five training blocks, significant group difference at end of training, * p < 0.05; chance level was 25%. b Mean percent of first choices to Constant 100% bowl locations during High-Variable sessions across five training blocks; chance level was 25%. c Mean percent of first choices to Low-Variable 25% bowl locations during Low-Variable sessions across five training blocks, significant group difference at beginning of training, ** p < 0.01; chance level was 25%. d Mean percent of first choices to Constant 100% bowl locations during Low-Variable sessions across five training blocks; chance level was 25%. e Mean percent of first choices to Incorrect bowl locations during both High-Variable and Low-Variable sessions across five training blocks, significant group difference at end of training, * p < 0.05; chance level was 50%. f Mean percent of first choices to both High-Variable 75% bowl locations and Low-Variable 25% bowl locations overall across five training blocks, significant group difference at end of training, * p < 0.05; chance level was 25%. Error bars indicate SEM. C control, HF hippocampal formation
Space Task: First choices to Low-Variable and Constant locations during Low-Variable trials
Visual inspection of Fig. 3c also suggests a notable difference between the C and HF pigeons, but here it was the C pigeons who were more likely to initially choose the less rational Low-Variable 25% bowl and change (decrease) their first choices to the Low-Variable 25% bowl across training. Mauchly’s test indicated that the assumption of sphericity had been violated χ2 (9) = 21.47, p = 0.01, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (e = 0.49). There was no main effect of blocked sessions F(1.95, 15.62) = 0.42, p = 0.66, but importantly, there was a robust main effect for group F(1, 8) = 14.16, p = 0.006. Additionally, there was not a significant interaction between blocked sessions and group F(1.95, 15.62) = 0.61, p = 0.56. The HF-lesion effect was already present early in training as a post hoc analysis revealed a significant between-group difference (BLOCK 1, C M = 43.75, SEM = 7.13; HF M = 11.25, SEM = 2.34; t(8) = 4.33, p = 0.003). As with the High-Variable trials, visual inspection of Fig. 3d suggests that there was no effect of HF lesions on first choices to the Constant 100% bowl during Low-Variable trials. There was a main effect of blocked sessions F(4,32) = 3.82, p = 0.01 as the pigeons increased their first choices to the Constant 100% bowl across training. However, there was no main effect for group F(1, 8) = 0.04, p = 0.84 nor a significant interaction between blocked sessions and group F(4,32) = 1.07, p = 0.39.
Although statistically underpowered by the relatively small sample sizes, the data presented in Fig. 3 suggests that the HF lesions affected the choice performance of the pigeons. There appear to be two effects of the lesions that we subsequently looked at more carefully. First, the data appear to reveal that the HF pigeons committed more space errors during training. Second, independent of High-Variable and Low-Variable trials, the HF pigeons appeared to make fewer choices to the food bowls associated with a variable outcome (75% or 25%), i.e., they seemed more risk averse.
Space Task: First-choice errors during High- and Low-Variable combined trials
As suggested above, visual inspection of Fig. 3e supports the observation that across training the HF pigeons made more first-choice errors by selecting a bowl location that never contained food (incorrect bowls). There was a significant main effect of blocked sessions F(4, 32) = 7.30, p < 0.001 as the pigeons made fewer errors across training. Importantly, there was an effect of group F(1,8) = 4.57, p = 0.07. There was no interaction effect between blocked sessions and group F(4, 32) = 1.05, p = 0.40. A post hoc test indicated that at the end of training the HF pigeons were making more first choice errors as there was a significant between-group difference (BLOCK 5, C M = 9.38, SEM = 4.64; HF M = 35.43, SEM = 10.12; t(8) = -2.34, p = 0.047). The significant group difference during Block 5 suggests a perhaps unsurprising spatial memory impairment following HF lesions; HF pigeons chose incorrect bowls significantly more often than C animals independent of High or Low Variable trial types.
Space Task: Pooled first choices to High- and Low-Variable locations
As suggested above, visual inspection of Fig. 3f further supports the observation that across training the HF pigeons, compared to the C birds, were less likely to preferentially select riskier food bowls (75% or 25% variable outcomes) as their first choice in comparison to bowl locations associated with a constant outcome (100%). There was not a significant main effect of blocked sessions F(4, 32) =0.84, p = 0.51. Importantly, there was a robust main effect for group F(1,8) = 12.17, p = 0.008 as the HF pigeons made fewer overall first choices to a variable (75% or 25%) reward food bowls. There was not a significant interaction between blocked sessions and group F(4, 32) = 0.50, p = 0.74. A post hoc analysis demonstrated that at the end of training there was a significant between-group difference (BLOCK 5, C M = 41.25, SEM = 6.43; HF M = 18.13, SEM = 5.54; t(8) = 2.72, p = 0.03).
In summary, the analyses on the number of errors and the number of variable food bowl choices suggest that the HF lesions resulted in impaired spatial learning, and more intriguingly, a shift away from choosing food bowls with variable outcomes; i.e., the HF pigeons seemed to become more risk averse.
First-choice errors: Space versus feature
Although not the main focus of the current study, we found it curious that the C pigeons trained to the Feature Task appeared to outperform the C pigeons trained to the Space Task (Figs. 2 and 3). To explore this observation further, we compared first-choice errors across training between the two groups of C pigeons. Note that because the Feature C pigeons were only trained for 16 sessions (four blocks of four sessions), only the first 16 sessions of the Space C pigeon data were used in the comparison. Inspection of Fig. 4 readily reveals that even though both groups reduced the number of their first-choice errors across training, the Feature pigeons (n = 8) clearly made fewer errors than Space birds (n = 5). Mauchly’s test indicated that the assumption of sphericity had been violated χ2 (5) = 16.474, p = 0.01, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (e = 0.58). There was a main effect of blocked sessions F(1.74, 19.13) = 17.57, p = 0.001 and, more importantly, group F(1, 11) = 25.56, p = 0.001. Additionally, there was not a significant interaction between blocked sessions and group F(1.74, 19.13) = 1.04, p = 0.37. A post hoc analysis revealed that at the end of training there was a significant between-group difference (BLOCK 4, Space M = 13.75, SEM = 4.15; Feature M = 0.39, SEM = 0.39; t(11) = 4.14, p = 0.002). At the end of training, Feature C birds hardly made any errors, whereas this was not the case for Space C birds.
Incorrect learning progression of the probability discrimination task by Space and Feature birds. Mean percent of first choices to Incorrect bowls (colors or locations) during both High-Variable and Low-Variable sessions across four training blocks, significant group difference at the end of training, ** p < 0.01; chance level was 50%. Error bars indicate SEM
Hippocampal-lesion reconstruction summary
The bilateral electrolytic HF-lesion damage sustained by the HF-lesioned Feature (Fig. 5a) and Space (Fig. 5b) birds are summarized in Fig. 5. All pigeons (Feature n = 7, Space n = 5) sustained extensive damage to the hippocampus proper (HP), with less damage to the neighboring parahippocampus (APH). Some animals also sustained minor damage to the adjacent hyperpallium apicale and mesopallium (M) on both sides.
Schematic coronal sections of the lesion reconstructions at 1.0-mm intervals from anterior (A 9.5) to posterior (A 4.5) according to the atlas of Karten and Hodos (1967), labeled according to the revised nomenclature (Reiner et al., 2004). a Lesion reconstructions of the HF Feature birds. The black areas represent damage seen in at least six of the seven pigeons with bilateral HF lesions. Gray areas represent damage seen in at least three of the seven pigeons with bilateral HF lesions. b Lesion reconstructions of the HF Space birds. The black areas represent damage seen in at least four of the five pigeons with bilateral HF lesions. Gray areas represent damage seen in at least two of the five pigeons with bilateral HF lesions. All pigeons sustained substantial damage to the hippocampus proper, with more variable damage to the neighboring parahippocampus. APH parahippocampus; CDL corticoid; E entopallium; HD hyperpallium densocellulare; HF hippocampal formation, HP hippocampus; M mesopallium; N nidopallium; V ventriculus
Discussion
A homing pigeon behaving as a rational agent should have preferentially chosen the high probability over the constant reward, and the constant over the low probability reward. Examination of Figs. 2 and 3 reveals that the C pigeons, as well as the HF-lesioned pigeons when tested with feature, generally behaved as rational agents. This is reflected in their preference for the High-Variable over the constant reward, and the constant over the Low-Variable reward. Under the conditions of the current study, C pigeons were not risk averse nor were HF-lesioned pigeons risk averse when reward discrimination was based on feature (see also Ludvig et al., 2014; Smith et al., 2017; Young, 1981). However, the interesting result is the difference in the preference profile of the C and HF-lesioned pigeons when the different reward outcomes were associated with different locations in space.
The procedures of the experiment were designed to offer an exploratory investigation of the possible importance of HF for decision making in the context of reward and risk. Given the association of HF with spatial cognition, we expected that HF lesions would have no effect on pigeons' choice behavior when goal locations were discriminated based on color. As expected, examination of Fig. 2 reveals no performance difference during the Feature Task between C and HF-lesioned birds. This finding compares well with previous avian work suggesting no evidence for HF control of non-spatial behavior (Coppola et al., 2014; Fremouw et al., 1997; Hampton & Shettleworth, 1996; Kahn & Bingman, 2009).
While we acknowledge the low statistical power, low subject sample size, and the generally exploratory nature of the study, there was nonetheless strong evidence pointing to a significant effect of HF lesions on the Space Task. Figure 3e showcases a significant difference between C and HF-lesioned birds regarding incorrect first choices. C pigeons were more likely to choose correct locations, whereas HF-lesioned pigeons made many more errors. This finding is not surprising and is consistent with numerous avian studies demonstrating spatial-memory deficits in remembering the location of food goals following HF lesions (e.g., Hampton & Shettleworth, 1996; Lormant et al., 2020; Strasser & Bingman, 1999).
The novel finding emerging from Fig. 3 is that the HF-lesioned birds were less likely to choose variable bowls (75% and 25%) in comparison to C pigeons. The data of Fig. 3f, along with Fig. 3a, suggest that the HF pigeons were less likely to direct their choices toward the riskier/variable options in comparison to the C pigeons. An impaired ability of HF-lesioned pigeons to use differential reward probabilities has been demonstrated elsewhere (Scarf et al., 2014). Our results are also consistent with previous studies related to goal-directed spatial behavior that suggested rodents with inactivated hippocampi routinely seek out stable, reliable, and constant reward-related outcomes compared to variable or risker options (Mizumori & Tyron, 2015; Mizumori et al., 2009; Mizumori et al., 2004; Wiener, 1993), as well as the more general observation of reward properties influencing hippocampal representations of space (Jeong et al., 2018; Jin & Lee, 2021; Jung et al., 2018; Sosa & Giocomo, 2021; Wood et al., 2000).
However, if there was a change towards becoming more risk averse due to HF lesions, it is not a general change in temperament as there was no hint of an aversion for riskier choices when the discrimination was based on features/color (Fig. 2). One explanation for the preference of the HF pigeons for the more predictable/less risky Constant (100%) food bowl location when reliant on memory for space is that the damage to HF resulted in “disorientation” with respect to space. We speculate that the disorientation may have increased stress (Kim & Leem, 2016; Lormant et al., 2021; Sandi, 2013; Sousa et al., 2000), thus promoting choices to the more predictable, constant food bowl. If our stress hypothesis can in part explain why the HF pigeons may have been more risk averse, one might expect differences in response latencies to first choices between the C and HF pigeons. However, we could detect no group difference in the response latency to first-choice data. The failure to detect a difference does not preclude a possible stress effect, and we suggest that examining bioindicators of stress such as corticosterone levels could prove illuminating. We would also like to again emphasize that any interpretation of our results should be considered provisional, awaiting follow-up experiments.
Peripheral to the main objective of the current study, it was nonetheless interesting that the C pigeons tested to feature outperformed the C pigeons tested to space. This result was surprising because in food-storing birds (Brodbeck, 1994; Brodbeck & Shettleworth, 1995; Clayton & Krebs, 1994; Sherry et al., 1992) and homing pigeons (Bingman et al., 2006; Nardi & Bingman, 2007; Vargas et al., 2004), there is typically a preference for spatial cues during goal localization or recognition, but not always (see Coppola et al., 2015; Kelly et al., 1998; LaDage et al., 2009; Maury et al., 2010; Strasser & Bingman, 1996). As revealed in Fig. 4, in comparison to the pigeons trained to space, the feature-trained birds were more likely to make correct first choices. These findings seem out of alignment with previous bird studies that demonstrate a spatial preference when both cues are simultaneously available (see above), or better performance with spatial, rather than feature, control of goal-directed behavior (Sizemore & Bingman, 2016). This leads to the question, why did discrimination based on space yield worse performance? If one assumes that performance on the Space Task, in part controlled by the HF, was more difficult than the Feature Task, heightened stress associated with the Spatial Task may have impacted HF function (mammals – Kim & Leem, 2016; Sandi, 2013; Sousa et al., 2000; Japanese quail – Lormant et al., 2021), perhaps explaining in part the poorer performance of the pigeons trained to space. However, as noted above, we can offer no data supporting the idea that the Space Task was more stressful. Alternatively, the poorer performance of the C pigeons trained to location may reflect a greater tendency to engage in exploratory behavior when discriminating among goals using spatial compared to feature information. In other words, the poorer performance of the C pigeons trained to space was not a consequence of less robust memory, but rather, a greater propensity to sample all sites periodically, perhaps to better learn about any eventual changes in the spatial location of food rewards (Lester, 1984).
In summary, the current study offers a first suggestion that in homing pigeons, spatial memory that captures the risk and reward properties of locations in an environment, and the decisions made based on that memory, are in part controlled by the HF. Although preliminary and in need of independent confirmation, the results presented should expand the conversation on a possible broader functional profile of the avian HF in memory that includes a role beyond environmental spatial maps to include what might be called environmental “value maps” (see Jung et al., 2018).
References
Bingman, V. P., & Jones, T. J. (1994). Sun compass-based spatial learning impaired in homing pigeons with hippocampal lesions. Journal of Neuroscience, 14(11), 6687-6694.
Bingman, V. P., Hough II, G. E., Kahn, M. C., & Siegel, J. J. (2003). The homing pigeon hippocampus and space: In search of adaptive specialization. Brain, Behavior and Evolution, 62, 117-127.
Bingman, V.P., Erichsen, J. T., Anderson, J. D., Good, M. A., & Pearce, J. M. (2006). Spared feature-structure discrimination but diminished salience of environmental geometry in hippocampal-lesioned homing pigeons (Columba livia). Behavioral Neuroscience, 120, 835-841.
Bischof, H. J., Lieshoff, C., & Watanabe, S. (2006). Spatial memory and hippocampal function in a non-foodstoring songbird, the zebra finch (Taeniopygia guttata). Reviews in the Neurosciences, 17(1-2), 43-52.
Brodbeck, D. R. (1994). Memory for spatial and local cues: A comparison of a storing and a nonstoring species. Animal Learning & Behavior, 22(2), 119-133. https://doi.org/10.3758/BF03199912
Brodbeck, D. R., & Shettleworth, S. J. (1995). Matching location and color of a compound stimulus: Comparison of a food-storing and a nonstoring bird species. Journal of Experimental Psychology: Animal Behavior Processes, 21(1), 64-77. https://doi.org/10.1037/0097-7403.21.1.64
Bullock, D. H., & Bitterman, M. E. (1962). Probability-matching in the pigeon. American Journal of Psychology, 75(4), 634-639.
Clayton, N. S., & Krebs, J. R. (1994). Memory for spatial and object-specific cues in food-storing and non-storing birds. Journal of Comparative Physiology A, 174(3), 371-379. https://doi.org/10.1007/BF00240218
Clayton, N. S., & Krebs, J. R. (1995). Memory in food-storing birds: from behavior to brain. Current Opinion in Neurobiology, 5, 149-154.
Colombo, M. & Broadbent, N. (2000). Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neuroscience and Biobehavioral Reviews, 24, 465-484.
Coppola, V. J., & Bingman, V. P. (2020). c-Fos revealed lower hippocampal participation in older homing pigeons when challenged with a spatial memory task. Neurobiology of Aging, 87, 98-107.
Coppola, V. J., Spencer, J. M., Peterson, R. M., & Bingman, V. P. (2014). Hippocampal lesions in homing pigeons do not impair feature-quality or feature-quantity discrimination. Behavioural Brain Research, 260, 83-91. https://doi.org/10.1016/j.bbr.2013.11.038
Coppola, V. J., Flaim, M. E., Carney, S. N., & Bingman, V. P. (2015). An age-related deficit in spatial-feature reference memory in homing pigeons (Columba livia). Behavioral Brain Research, 280, 1-5, https://doi.org/10.1016/j.bbr.2014.11.026
Essock, S. M., & Reese, E. P. (1974). Preference for and effects of variable as opposed to fixed-reinforcer duration. Journal of Experimental Analysis of Behavior, 21, 89-97.
Fremouw, T., Jackson-Smith, P., & Kesner, R. P. (1997). Impaired place learning and unimpaired cue learning in hippocampal-lesioned pigeons. Behavioral Neuroscience, 111(5), 963.
Graf, V., Bullock, D. H., & Bitterman, M. E. (1964). Further experiments on probability-matching in the pigeon. Journal of the Experimental Analysis of Behavior, 7, 151–157. https://doi.org/10.1901/jeab.1964.7-151
Hamm, S. L., & Shettleworth, S. J. (1987). Risk aversion in pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 13(4), 376-383.
Hampton, R. R., & Shettleworth, S. J., (1996). Hippocampus and memory in a food-storing and in a nonstoring bird species. Behavioral Neuroscience, 110, 946-964.
Herold, C., Coppola, V. J., Bingman, V. P., 2015. The maturation of research into the avian hippocampal formation: Recent discoveries from one of the nature’s foremost navigators. Hippocampus, 25, 1193-1211.
Hough, G. E., & Bingman, V. P. (2004). Spatial response properties of homing pigeon hippocampal neurons: correlations with goal locations, movement between goals, and environmental context in a radial-arm arena. Journal of Comparative Physiology A, 190, 1047-1062.
Jeong, Y., Huh, N., Lee, J., Yun, I., Lee, J. W., Lee, I., & Jung, M. W. (2018). Role of the hippocampal CA1 region in incremental value learning. Scientific Reports, 8, 1-15.
Jin, S. W., & Lee, I. (2021). Differential encoding of place value between the dorsal and intermediate hippocampus. Current Biology 31, 3053-3072. https://doi.org/10.1016/j.cub.2021.04.073.
Johnston, M., Scarf, D., Wilson, A., Millar, J., Bartonicek, A., & Colombo, M. (2021). The effects of hippocampal and area parahippocampalis lesions on the processing and retention of serial-order behavior, autoshaping, and spatial behavior in pigeons. Hippocampus, 31, 261-280. https://doi.org/10.1002/hipo.23287
Jung, M. W., Lee, H., Jeong, Y., Lee, J. W., & Lee, I. (2018). Remembering rewarding futures: A simulation-selection model of the hippocampus. Hippocampus, 28(12), 913-930.
Kahn, M. C., & Bingman, V. P. (2009). Avian hippocampal role in space and content memory. European Journal of Neuroscience, 30, 1900-1908.
Kahn, M. C., Siegel, J. J., Jechura, T. J., & Bingman, V. P. (2008). Response properties of avian hippocampal formation cells in an environment with unstable goal locations. Behavioural Brain Research, 191, 153-163.
Karten, H. J., & Hodos, W. (1967). A stereotaxic atlas of the brain of the pigeon (Columba livia). John Hopkins Press.
Kelly, D. M., Spetch, M. L., & Heth, C. D. (1998). Pigeons’ (Columba livia) encoding of geometric and featural properties of a spatial environment. Journal of Comparative Psychology, 112(3), 259-269.
Kim, D. M., & Leem, Y. H. (2016). Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience, 324, 271-285. https://doi.org/10.1016/j.neuroscience.2016.03.019
LaDage, L. D., Roth II, T. C., Fox, R. A., & Pravosudov, V. V. (2009). Flexible cue use in food-caching birds. Animal Cognition, 12(3), 419-426. https://doi.org/10.1007/s10071-008-0201-0
Lagorio, C. H., & Hackenberg, T. D. (2012). Risky choice in pigeons: Preference for amount variability using a token-reinforcement system. Journal of the Experimental Analysis of Behavior, 98, 139-154. https://doi.org/10.1901/jeab.2012.98-139
Lester, N. P. (1984). The “feed:feed” decision: How goldfish solve the patch depletion problem. Behaviour, 89(3/4), 175-199.
Lormant, F., Cornilleau, F., Constantin, P., Meurisse, M., Lansade, L., Leterrier, C., Levy, F., & Calandreau, L. (2020). Role of the hippocampus in spatial memory in Japanese quail. Poultry Science, 99, 61-66.
Lormant, F., Ferreira, V. H. B., Lemarchand, J., Cornilleau, F., Constantin, P., Parias, C., Bertin, A., Lansade, L., Leterrier, C., Levy, F., & Calandreau, L. (2021). Training levels reveals a dynamic dialogue between stress and memory in birds. Behavioral Brain Research, 408, 113280. https://doi.org/10.1016/j.bbr.2021.113280.
Ludvig, E. A., Madan, C. R., Pisklak, J. M., & Spetch, M. L. (2014). Reward context determines risky choice in pigeons and humans. Biology Letters, 10, 20140451. https://doi.org/10.1098/rsb.2014.0451
Mamad, O., Stumpp, L., McNamara, H. M., Ramakrishnan, C., Deisseroth, K., Reilly, R. B., & Tsanov, M. (2017). Place field assembly distribution encodes preferred locations. PLoS Biology, 15(9), e2002365. https://doi.org/10.1371/journal.pbio.2002365
Maury, D. L., Mauch, R. J., Hammer, A. N., & Bingman, V. P. (2010). Spatial and feature-based memory representation in free-flying homing pigeons. Animal Cognition, 13(5), 733-743. https://doi.org/10.1007/s10071-010-0324-y
Mayer, U., & Bischof, H. J. (2012). Brain activation pattern depends on the strategy chosen by zebra finches to solve an orientation task. Journal of Experimental Biology, 215(3), 426-434.
Mayer, U., Watanabe, S., & Bischof, H. J. (2010). Hippocampal activation of immediate early genes Zenk and c-Fos in zebra finches (Taeniopygia guttata) during learning and recall of a spatial memory task. Neurobiology of Learning and Memory, 93(3), 322-329.
Mayer, U., Pecchia, T., Bingman, V. P., Flore, M., & Vallortigara, G. (2016). Hippocampus and medial striatum dissociation during goal navigation by geometry or features in the domestic chick: An immediate early gene study. Hippocampus, 26(1), 27-40.
Mayer, U., Bhushan, R., Vallortigara, G., & Lee, S. A. (2018). Representation of environmental shape in the hippocampus of domestic chicks (Gallus gallus). Brain Structure and Function, 223, 941-953.
Menlove, R. L., Inden, H. M., & Madden, E. G. (1979). Preference for fixed over variable access to food. Animal Learning & Behavior, 7(4), 499-503.
Mizumori, S. J. Y., & Tyron, V. L. (2015). Integrative hippocampal and decision-making neurocircuitry during goal-relevant predictions and encoding. Progress in Brain Research, 219, 217-241.
Mizumori, S. J. Y., Yeshenko, O., Gill, K. M., & Davis, D. M. (2004). Parallel processing across neural systems: Implications for a multiple memory system hypothesis. Neurobiology of Learning and Memory, 82, 278-292. https://doi.org/10.1016/j.nlm.2004.07.007
Mizumori, S. J. Y., Puryear, C. B., & Martig, A. K. (2009). Basal ganglia contributions to adaptive navigation. Behavioural Brain Research, 199(1), 32-42. https://doi.org/10.1016/j.bbr.2008.11.014
Nardi, D., & Bingman, V. P. (2007). Asymmetrical participation of the left and right hippocampus for representing environmental geometry in homing pigeons. Behavioral Brain Research, 178, 160-171.
Payne, H. L., Lynch, G. F., & Aronov, D. (2021). Neural representations of space in the hippocampus of a food-caching bird. Science, 373, 343-348.
Penner, M. R., & Mizumori, S. J. Y. (2012). Neural systems analysis of decision making during goal-directed navigation. Progress in Neurobiology, 96, 96-135. https://doi.org/10.1016/j.pneurobio.2011.08.010
Pravosudov, V. V., & Smulders, T. V. (2010). Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 365(1542), 859-867. https://doi.org/10.1098/rstb.2009.0216
Reiner, A., Perkel, D. J., Bruce, L. L., Butler, A. B., Csillag, A., Kuenzel, W., et al. (2004). Revised nomenclature for avian telencephalon and some related brainstem nuclei. Journal of Comparative Neurology, 473, 377-414.
Retailleau, A., Etienne, S., Guthrie, M., & Boraud, T. (2012). Where is my reward and how do I get it? Interaction between the hippocampus and the basal ganglia during spatial learning. Journal of Physiology – Paris, 106, 72-80. https://doi.org/10.1016/j.jphysparis.2011.10.002
Roberts, W. A., Strang, C., Macpherson, K. (2015). Memory systems interaction in the pigeon: Working and reference memory. Journal of Experimental Psychology: Animal Learning and Cognition, 41, 152-12. https://doi.org/10.1037/xan0000053
Roberts, W. A., MacDonald, H., & Ho Lo, K. (2018). Pigeons play the percentages: Computation of probability in a bird. Animal Cognition, 21, 575-581. https://doi.org/10.1007/s10071-018-1192-0
Sandi, C. (2013). Stress and cognition. WIREs Cognitive Science, 4, 245-261.
Scarf, D., Millar, J., Pow, N., & Colombo, M. (2014). Inhibition, the final frontier: The impact of hippocampal lesions on behavioral inhibition and spatial processing in pigeons. Behavioral Neuroscience, 128(1), 42-47. https://doi.org/10.1037/a0035487
Sherry, D. F., & Vaccarino, A. L. (1989). Hippocampus and memory for food caches in black-capped chickadees. Behavioral Neuroscience, 103, 308.
Sherry, D. F., Vaccarino, A. L., Buckenham, K., & Herz, R. S. (1989). The hippocampal complex of food-storing birds. Brain, Behavior and Evolution, 34(5), 308-317. https://doi.org/10.1159/000116516
Sherry, D. F., Jacobs, L. F., & Gaulin, S. J. (1992). Spatial memory and adaptive specialization of the hippocampus. Trends in Neurosciences, 15(8), 292-303. https://doi.org/10.1016/0166-2236(92)90080-R
Shiflett, M. W., Smulders, T. V., Benedict, L., & DeVoogd, T. J., (2003). Reversible inactivation of the hippocampal formation in food-storing black-capped chickadees (Poecile atricapillus). Hippocampus, 13, 437-444.
Siegel, J. J., Nitz, D., & Bingman, V. P. (2005). Spatial-specificity of single-units in the hippocampal formation of freely moving homing pigeons. Hippocampus, 15(1), 26-40.
Sizemore, B. A., & Bingman, V. P. (2016). Time-of-day discriminative learning: Contrasting the use of spatial compared to feature information in homing pigeons (Columba livia). Ethology, 122, 982-990. https://doi.org/10.1111/eth.12569.
Smith, A. P., Beckmann, J. S., & Zentall, T. R. (2017). Gambling-like behavior in pigeons: “Jackpot” signals promote maladaptive risky choice. Scientific Reports, 7, 6625. https://doi.org/10.1038/s41598-017-06641-x
Smulders, T. V. (2006). A multi-disciplinary approach to understanding hippocampal function in food-hoarding birds. Reviews in the Neurosciences, 17, 53-69.
Smulders, T. V., & DeVoogd, T. J. (2000). Expression of immediate early genes in the hippocampal formation of the black-capped chickadee (Poecile atricapillus) during a food-hoarding task. Behavioural Brain Research, 114(1-2), 39-49.
Sosa, M., & Giocomo, L. M. (2021). Navigating for reward. Nature Reviews Neuroscience, 22, 472-487.
Sousa, N., Lukoyanov, N. V., Madeira, M. D., Almeida, O. F. X., & Paula-Barbosa, M. M. (2000). Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience, 97(2), 253-266
Staddon, J. E. R., & Innis, N. K. (1966). Preference for fixed vs. variable amounts of reward. Psychonomic Science, 4, 193-194.
Strasser, R., & Bingman, V. P. (1996). The relative importance of location and feature cues for homing pigeon (Columba livia) goal recognition. Journal of Comparative Psychology, 110, 77-87.
Strasser, R., & Bingman, V. P. (1997). Goal recognition and hippocampal formation in the homing pigeon (Columba livia). Behavioral Neuroscience, 111, 1245-1256.
Strasser, R., & Bingman, V. P. (1999). The effects of hippocampal lesions in homing pigeons on a one-trial food association task. Journal of Comparative Physiology A, 185, 583-590.
Tryon, V. L., Penner, M. R., Heide, S. W., King, H. O., Larkin, J., & Mizumori, S. J. Y. (2017). Hippocampal neural activity reflects the economy of choices during goal-directed navigation. Hippocampus, 27(7), 1-16. https://doi.org/10.1002/hipo.22720
Vargas, J. P., Petruso, E. J., & Bingman, V. P. (2004). Hippocampal formation is required for geometric navigation in pigeons. European Journal of Neuroscience, 20(7), 1937-1944. https://doi.org/10.1111/j.1460-9568.2004.03654.x
Wiener, S. I., (1993). Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. The Journal of Neuroscience, 13(9), 3802-3817.
Wood, E. R., Dudchenko, P. A., Robitsek, R. J., & Eichenbaum, H. (2000). Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron, 27, 623-633.
Young, J. S. (1981). Discrete-trial choice in pigeons: Effects of reinforcer magnitude. Journal of Experimental Analysis of Behavior, 65, 23-29.
Acknowledgments
The authors would like to thank Catherine E. Petrowski for helpful suggestions on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
None
Additional information
Open Practices Statement
The data or materials for the experiments reported here are available from the corresponding author on reasonable request. None of the experiments were preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sizemore, B.A., Bausher, A., Paul, E. et al. Space, feature, and risk sensitivity in homing pigeons (Columba livia): Broadening the conversation on the role of the avian hippocampus in memory. Learn Behav 50, 99–112 (2022). https://doi.org/10.3758/s13420-021-00500-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-021-00500-6