Abstract
Social emotions require the correct integration of emotional, cognitive, and social processes and are critical for complex social interactions. Adolescent criminal offenders (AOs) show abnormalities in the experience of basic emotions. However, most research has focused solely on basic emotions, neglecting complex social emotions that could be critical for social reintegration. The purpose of this study was to investigate the behavioral and neural correlates of social emotions (envy and Schadenfreude) in AOs. We explored the experience of complex social emotions, as well as their anatomical correlates, in AOs (n = 19) and a nonoffenders control group (NOs, n = 20). Additionally, we assessed the relationship between social emotions, executive functions (EFs), and fluid intelligence (FI). Structural brain imaging was obtained in all participants. The results showed that AOs had significantly lower envy and Schadenfreude ratings and exhibited lower performance in EFs compared with NOs. The measurement of EFs relied on the INECO frontal screening (IFS). Experiencing fewer social emotions was associated with diminished EFs but not with FI. Moreover, in AOs, reduced levels of envy and Schadenfreude were linked with reduced gray matter volumes in regions subserving mentalizing abilities (inferior parietal lobe and precuneus) and socioemotional processing (inferior and middle temporal regions), as well as key hubs of the executive frontoparietal network (inferior parietal lobule, orbital and rectus gyri). Additional analysis on the AOs revealed no associations between the type of crime and our variables of interest (EFs, FI and social emotions). Our findings are the first to provide evidence on abnormalities in the experience of social emotions in AOs that are associated with neurocognitive markers of social cognition and EFs. Understanding social emotions and their abnormalities (under-experience) as complex intertwined processes may have important future translational implications, including risk prediction for social adaptation/reintegration, sociocognitive targeted interventions, and skill training for social emotions in vulnerable populations.
Similar content being viewed by others
Introduction
Complex social emotion abilities are critical for life in society and social interaction (Frith, 2001; Li et al., 2017; Ouwerkerk et al., 2018; Paulus et al., 2018; Qi et al., 2020). Insufficiencies in these abilities may result in social adaptation issues and nonprosocial behaviors (Jusyte & Schönenberg, 2017). In fact, adolescent criminal offenders (AOs) show multiple socioemotional impairments (Jones et al., 2007; Palmer & Hollin, 1999), such as poor facial emotion recognition (particularly anger and disgust) (Bowen et al., 2014; Jones et al., 2007), as well as difficulties in detecting gaze direction (Jones et al., 2007). Inability to recognize gaze direction may set the path for the maladaptive development of social abilities (Jones et al., 2007) while emotion recognition flaws may partially explain why AOs react inappropriately to social interactions. Poor emotion recognition has been consistently linked to violent and criminal behavior. For instance, impaired emotion recognition facilitates violent behavior and recidivism in criminal offenders (Leshem et al., 2019). In addition, research has shown that subsequent crime was greatly reduced by improving facial emotion recognition in offenders (Hubble et al., 2015; Kuin et al., 2019; Timmermann et al., 2017). However, most previous evidence has focused on basic emotions, and no study to date has investigated complex social emotions.
Social emotions follow societal and moral rules, ultimately serving the interest and welfare of individuals or societies (Haidt, 2003; Tangney et al., 2007). These types of emotions motivate adherence to societal rules and foster behaviors that are beneficial to others while deterring those that are harmful or damaging. Social emotions differ from basic emotions in that the former recruits a machinery that support complex cognitive processes, such as empathy, perspective taking, self-processing, and reward processing (Jankowski & Takahashi, 2014). Such processes imply the acknowledgment of the content of others minds, as well as social comparison. Comparison with others and thoughts about others expectations are necessary for social emotions but not for basic emotions.
Social emotions can be prosocial or antisocial, depending on the social context (Shamay-Tsoory et al., 2014). This is the case of envy and Schadenfreude–feeling displeasure/pleasure associated with another’s fortunes/misfortunes (Jankowski & Takahashi, 2014). These are complex multidimensional emotions based on social comparison (Shamay-Tsoory et al., 2014). Such social comparison relies on cognitive (ToM, mentalizing), emotional (empathy) (Baez, Pino, et al., 2018b; Chester et al., 2013; Santamaría-García et al., 2017), and higher cognitive abilities, such as executive functions (EFs) (Santamaría-García et al., 2017). Furthermore, these social emotions can be evoked from a variety of social contextual factors (Shamay-Tsoory et al., 2014). For instance, observing certain outcomes after moral, societal, or legal rule breaches may trigger the experience of social emotions that could motivate actions for social cohesion (Vaish, 2018). Moreover, experiences of envy and Schadenfreude can arise from inconformity with an illegitimate gain of another individual (Smith et al., 1996; Van Dijk et al., 2006). However, envy and Schadenfreude can be evoked by self-focused processes related to individual feelings of inferiority regardless of the context (Leach et al., 2003) or by other focused processes, such as encountering an individual who is successful in a desired domain (Smith, 1991; Tajfel & Turner, 1979).
Studies assessing the structural correlates of envy have associated this feeling with the gray matter (GM) volume in the anterior/mid cingulate cortex (ACC and MCC) and temporal areas (Santamaría-García et al., 2017). Schadenfreude is associated with hubs of reward (ventral striatum) and mentalizing (precuneus and the superior parietal lobule) networks (Baez, Pino, et al., 2018b; Dvash et al., 2010; Jankowski & Takahashi, 2014; Mendez, 2009; Paulus et al., 2018; Rademacher et al., 2010; Spreckelmeyer et al., 2009; Takahashi et al., 2009). Another region associated with both envy and Schadenfreude is the insular (Jankowski & Takahashi, 2014). More specifically, the insular cortex integrates perceptive, emotional, and reasoning areas to create the sense of an interpersonal world (Kurth et al., 2010). Particularly, the anterior portion of the insular is associated with social comparison, the process by which individuals project their personal preferences onto others (Steinbeis & Singer, 2014). In addition, frontotemporal regions have been shown to be important modulators of both envy and Schadenfreude in patients with behavioral variants of frontotemporal dementia, Alzheimer’s disease, and healthy participants (Santamaría-García et al., 2017).
These brain regions (Cai & Liu, 2004; Sedeño et al., 2016; Seeley et al., 2012) also have been associated with EFs. EFs play a role in envy and Schadenfreude experiences (Santamaría-García et al., 2017), specifically, decreased executive control and a lack of inhibition have been associated with issues in the experience of both emotions. Previous research shows that inhibitory control and working memory are necessary to understand the mental states of oneself and others (Carlson et al., 2002; Carlson & Moses, 2001). Inhibiting own perspectives may be important to understand that of others (Carlson & Moses, 2001) while working memory serves to consider various perspectives at a given moment. Further research has shown that the strategic control of thoughts and behavior during social interactions are strongly related to ToM tasks (Ferner & Lang, 1999; Lang & Perner, 2002). Indeed, neuroimaging research has shown concurrent abnormalities in a common architecture subserving both EFs and ToM abilities in individuals with socioemotional impairment (i.e., autism) (Ellis & Gunter, 1999; Ozonoff et al., 1991; Wade et al., 2018). Thus, lowered EFs affecting ToM may compromise the experience of envy and Schadenfreude by disabling the social comparison processes that would ultimately trigger such emotions (Smith et al., 1996). For instance, failure to maintain perspectives of oneself and others in working memory may impair the appropriate integration of one state relative to others states, which would weaken the experience of envy and Schadenfreude. Similarly, failure to inhibit one’s perspective leads in undermining fortunes/misfortunes of others, resulting in blunted envy/Schadenfreude. In fact, research on clinical populations has shown that individuals with or prone to Huntington’s disease (which also exhibit poor ToM performance) show decreased levels of envy and Schadenfreude (Baez et al., 2016b). These findings imply that subjects with lower performance in EFs may show a diminished experience of envy and Schadenfreude.
Low performances in EFs as well as fluid intelligence (FI) tests have been consistently reported in AOs (Bergeron & Valliant, 2001; Kelly et al., 2002; Veneziano et al., 2004; Villemarette-Pittman et al., 2003). FI is defined as the use of controlled mental operations to solve novel problems that cannot be performed automatically (McGrew, 2009). Reported difficulties in EFs include underperformance in planning (Syngelaki et al., 2009; Zou et al., 2013), attentional shifting (Zou et al., 2013), working memory (Syngelaki et al., 2009; Zou et al., 2013), cognitive flexibility, and task monitoring (Burton et al., 2016). With that in mind, it would be expected that AOs will exhibit a blunted or diminished experience of envy and Schadenfreude compared with controls. This under-experience would be related to lowered EFs. However, to date, no previous research has explored this issue.
Considering this background, this work was designed to assess social emotional experiences (envy and Schadenfreude) and their structural correlates in a group of AOs compared with those in control subjects. We also investigated the relationship between social emotions and basic cognitive domains (i.e., EFs and FI). Taking into account that AOs show lower levels of emotion recognition, mentalizing and empathic abilities (all prerequisites of envy and Schadenfreude) (Mariano et al., 2017), we expect that social emotional experience also will be lower compared with controls. We also make this prediction based on the notion that the inability to understand others’ mental states hinders social comparison and consequently any emotional reaction from an observed event. In fact, previous research has linked ToM issues to diminished experience of envy and Schadenfreude in clinical populations (Baez, Pino, et al., 2018b; Baez et al., 2016b; García-Cordero et al., 2016). Given that EFs play an important role in mentalizing abilities (Smith et al., 1996), we also expect that low performance of EFs in AOs will be associated with under experience of envy and Schadenfreude. Additionally, considering that previous studies in AOs have shown differences in FI (Gonzalez-Gadea et al., 2014; Holland et al., 2002; Ttofi et al., 2016) and the reported the association between EFs and FI (Rey-Mermet, Gade, Souza, von Bastian, & Oberauer, 2019), we considered relevant to assess this domain in our study. However, the association between FI and emotion recognition and experience is not as clear-cut. Indeed, findings have been contradictory regarding the relationship between these variables (Bardeen et al., 2013; De Stasio et al., 2014; Shamosh & Gray, 2007). Indeed, there seems to be a disconnection between EFs and FI in explaining criminal behaviors (Herrero et al., 2019). Considering this, we expected no association between social emotions and FI in AOs. Finally, with regards to neural correlates of social emotions, we predict that AOs’ atypical social emotion processing will be associated with reduced GM volumes in brain areas involved in social cognition (Baez et al., 2017a; Chester et al., 2013) and EFs (Santamaría-García et al., 2017).
Methods
Participants
Thirty-nine male subjects between aged 14 and 19 years were recruited for this study. We used this age range (adolescence), because it is a key period for the development of cognitive and socioemotional processes (Blakemore & Choudhury, 2006; Yurgelun-Todd, 2007).
From this sample, 19 were AOs [age (M = 17.47; SD = 1.50), years of education (M = 9.10; SD = 2.05)] and 20 were control subjects who had not committed an offense [age (M = 16.85; SD = 1.66), years of education (M = 9.55; SD = 1.14)]. Both groups were matched age and years of education (Table 1). Educational level was measured by years of education and was obtained through school archives for NOs and by the reinsertion center were NOs were being held. Similarly, criminal history was provided by the institution where AOs were held. The offender population was recruited from a reeducation and social reinsertion center for young male offenders in Barranquilla, Colombia. Offenses from these subjects ranged from theft (qualified or aggravated, 26.31%), homicide attempt (5.26%), homicide (52.6%), extortion (5.26%), violent sexual access (5.26%), and illegal carry of a weapon (5.26%) (Table 1).
The NOs sample was recruited from schools located in the same area where offenders lived. To ensure that they had not committed any criminal offense in the past, we conducted an interview with the participants as well as their parents in which we asked for potential criminal history. All participants completed a screening test to rule out the possibility of psychiatric/neurological disorders or current pharmacological treatment. In line with ethical guidelines, recruitment was performed after obtaining consent from the principal and teachers from each institution. All participants and parents provided written, informed assent/consent in agreement with the Helsinki declaration. In the cases where informed consent was not obtained from the parents, the legal guardians provided the informed consent. The protocol was approved by the Ethics Committee of the Universidad Autónoma del Caribe.
Instruments
Executive functions and fluid intelligence
We assessed EFs and fluid intelligence, given its possible impact on emotion processing (Dodonova & Dodonov, 2012; Ibáñez et al., 2011; Yurgelun-Todd, 2007). EFs assessment was based on the INECO Frontal Screening test (IFS) (Torralva et al., 2009). The IFS instrument assesses distinct EFs, such as response inhibition, set shifting, working memory, and abstraction capacity. The IFS has been previously administered to both clinical and nonclinical populations (Baez, Couto, et al., 2014a; Baez, Herrera, et al., 2017b) as well as in AOs (Gonzalez-Gadea et al., 2014; Santamaría-García et al., 2019)
Response inhibition and set shifting were measured with four subscales:
-
Motor programming (3 points). In the motor series, patients first watched the administrator perform the Luria series (“fist, edge, palm”) and then performed it on their own. If the participant achieved six consecutive series by themselves, their score was 3; if they achieved at least three consecutive series, their score was 2; if they failed to achieve at least three consecutive series alone but achieved three when copying the examiner, their score was 1; otherwise, they would score 0.
-
Conflicting instructions (3 points). Subjects were asked to hit the table once when the administrator hit it twice and to hit the table twice when the administrator hit it once. After a practice trial, the examiner completed the following series: 1-1-2-1-2-2-1-1-2. If subjects made no errors, their score was 3; if subjects made one or two errors, their score was 2; and for more than two errors, their score was 1. Copying or imitating the examiner is a common behavior seen in frontal lesion patients. If the subject copied the examiner at least four consecutive times, their score was 0.
-
Go–No go task (3 points). Subjects were told to hit the table once when the examiner hit it once and to do nothing when the examiner hit it twice. After practice, the examiner completed the following series: 1-1-2-1-2-2-2-1-1-2. In the same way as in the conflicting instructions task, no mistakes meant 3 points, one or two errors was 2 points, more than two errors was a score of 1, and copying the examiner at least four consecutive times meant a score of 0.
-
Verbal inhibitory control (6 points). Subjects had to complete a sentence with a single word as quickly as possible. In the first part, 2 sentences were presented that strongly constrained what the missing word would be (i.e., “I put on my shoes and tie my__”). In the second part, the subject was asked to complete three sentences in a way that was syntactically correct but had no meaning (i.e., “an eye for an eye, a tooth for a __table__”). Only the second part of this task was scored. For each sentence, a score of 2 was given for a word that was unrelated to the sentence, a score of 1 was given for a word semantically related to the sentences, and a score of 0 was given for the expected word.
-
As for working memory testing, there were three subscales measuring central executive functions:
-
Backward digit span task (6 points). In the backward digit span task, subjects were asked to repeat progressively longer series of numbers in reverse order. The series started with two numbers and finished with eight numbers. If subjects failed to repeat a series, they were given an alternative series with the same number of digits. If the participant failed to repeat the alternative series, the task was discontinued. The score consisted of the number of digits that the subject was able to repeat (with a maximum of 6).
-
Verbal working memory subscale (2 points). The subject was asked to list the months of the year in reverse order, starting with December. If subjects made no errors, their score was 2, and for one error, their score was 1; otherwise, the score was 0.
-
Spatial working memory scale (4 points). The examiner presented four cubes and pointed at them in a given sequence. Subjects were asked to repeat each sequence in the reverse order. There were four sequences, starting with two blocks and increasing consecutively until four blocks were used. The score was the number of correctly completed sequences.
Finally, the abstraction capacity subscale (3 points) consisted of a proverb interpretation task in which participants were told three proverbs and were then asked to explain their meaning. For each adequate explanation, a score of 1 was given; a score of 0.5 was given when a correct example was provided. Otherwise, the score was 0.
It is important to mention that the IFS has previously been administered to both clinical and nonclinical populations (Baez, García, et al., 2017a; Baez, Ibanez, et al., 2014b), as well as in AOs (Gonzalez-Gadea et al., 2014; Santamaría-García et al., 2017). Additionally, increasing efforts show that IFS is useful for assessing EFs in healthy populations (Gómez-Carvajal et al., 2020; Sierra Sanjurjo et al., 2019) (see details in S1).
Fluid intelligence was assessed via Raven’s standard Progressive Matrices (Raven, 1960). The Raven’s progressive matrices (RPM) measures the ability to extract meaning and make associations from novel stimuli, or through reasoning (John & Raven, 2003). In the RPM, subjects have to complete increasingly difficult sets of figures, through the use of pattern recognition, logic, and abstraction (John & Raven, 2003). The RPM has been used as a standard measure of FI (gF) recruiting lateral prefrontal and parietal regions (Gray et al., 2003). It also has been repeatedly used to measure differences in general FI in a variety of populations (Paul, 1986; Roccatagliata & Benassi, 1981; Staff et al., 2014), including AOs (Donnellan et al., 2000; Santamaría-García et al., 2019; Sigurdsson & Gudjonsson, 1996; Van Wijk et al., 2005).
Social-emotion task
We used a social-emotion task previously reported in clinical (Baez, Pino, et al., 2018b; Baez et al., 2016b; Santamaría-García et al., 2017) and nonclinical populations (Santamaría-García et al., 2019). Studies on clinical populations revealed associations between Schadenfreude ratings and GM in key regions for mentalizing (precuneus) and reward processes (ventral striatum) (Baez, Pino, et al., 2018b; Baez et al., 2016b; Santamaría-García et al., 2017) while envy scores were positively associated with GM volumes in the ACC, the bilateral amygdala, and the parahippocampus. The task consisted of two blocks (one for envy and the other for Schadenfreude). Based on previous research (Jankowski & Takahashi, 2014; Takahashi et al., 2009) that suggests levels of envy predict the experience of Schadenfreude, the envy block was presented first, followed by the Schadenfreude block. Within each block, five situations describing neutral events were included. In the first block, participants read 15 sentences describing fortunate events involving 2 characters (e.g., “(s)he went on vacations with the money she saved by avoiding taxes”). After reading each sentence, participants rated the event in terms of how much displeasure (envy) they felt for the character. In the second block, participants read and reported the intensity of their pleasure (Schadenfreude) in response to 15 unfortunate events happening to the characters (e.g., “(s)he got discovered for trying to scam an elderly person”). A last set of 10 situations evoked neutral feelings. Neutral situations involved incidental scenarios that were not intended to evoke any feelings and resembled the following “(s)he turned off the light and closed the door before leaving home.” All participants provided their responses using a 9-point Likert scale, with one meaning “no pleasure/displeasure” and nine meaning “extreme pleasure/displeasure.” (For the complete social emotion task stimuli refer to supplementary material S1.)
To ensure that subjects comprehended the assessment, prior to the task, they were shown situations with positive, negative, and neutral outcomes affecting a third person and were then asked to determine how fortunate or unfortunate the situation was. After ensuring comprehension, we proceeded to administer the social emotion task. For this task, subjects were presented first with a real-life photograph and a description of two target characters matching their age and gender. The situations for each emotion occurred to the target character, and subjects had to rate their level of pleasure or displeasure in response to the situations. Subjects reported their intensity of displeasure (for the envy block) and pleasure (Schadenfreude block). The words displeasure and pleasure were used to measure experiences of envy and Schadenfreude, given that the former words are the overarching experiences associated with each emotion (i.e., envy is characterized by feelings of displeasure associated with another’s positive outcomes, while Schadenfreude is characterized by feelings of pleasure associated with unfortunate events or outcomes experienced by others).
It is important to note that in our task, participants were asked to report their Schadenfreude and envy in terms of pleasure/displeasure as these are the main overarching states elicited by each of those emotions (Santamaría-García et al., 2017). Previous research has defined envy as “an unpleasant, often painful emotion characterized by feelings of inferiority, hostility, and resentment caused by an awareness of a desired attribute by another person or group of persons” (Dvash et al., 2010; Jankowski & Takahashi, 2014; Smith et al., 2009; Smith & Kim, 2007). Schadenfreude also has been defined as “the pleasure derived from the misfortune of others,” involving “the expression of pleasure or self-satisfaction at another’s failure” (Dvash et al., 2010; Jankowski & Takahashi, 2014; Van Dijk et al., 2006). Therefore, we employed the terms “pleasure” and “displeasure,” highlighting the critical emotional responses elicited by specific scenarios of the assessed social emotions. Besides, explicit manifestations of envy and Schadenfreude are usually socially penalized (Dvash et al., 2010; Jankowski & Takahashi, 2014; Van Dijk et al., 2006) and, hence, people might report lower levels of these emotions due to social desirability. Our task procedure circumvents such biases by avoiding explicit questions, in line with previous methodological recommendations for exploring social and affective cognitive processes (Berkman et al., 2014). Indeed, asking about levels of pleasure/displeasure are the standard procedure as reported in previous studies of envy and Schadenfreude (Baez, García, & Santamaría-García, 2017a; Baez et al., 2016b; Santamaría-García et al., 2017; Takahashi et al., 2009). Finally, the terms “pleasure” and “displeasure” are easier to understand and thus more reliable than “envy” and “Schadenfreude” as verbally cued measures.
Imaging recordings
Participants were scanned in a 1.5 T Siemens MAGNETOM equipped with a standard head coil. The anatomical and 3D T1-weighted images had the following parameters: TR = 7.9, TE = 3.8, ACQ matrix 220 x 220 pixels, voxel size 0.5 x 0.5 x 0.5 mm, 310 sections.
Data analysis
To explore the association between EFs (IFS total score and subscales) and envy and Schadenfreude in the AOs group, we conducted correlation analyses
Behavioral data
Behavioral data were compared using ANOVA. Categorical variables (i.e., group) were compared via chi-square tests. Then, an ANCOVA model included the following variables: EFs, age, educational level, and social status. The latter variable refers to the socioeconomic status to which participants belong. Those with the lowest income belong to “status 1,” and those with the highest income belong to “status 6.” Only those effects that remained significant after covariation were reported.
We then conducted multiple regression analysis to explore the association between EFs and FI with social emotions. We estimated two models in which Schadenfreude and envy ratings were considered dependent variables. The following variables were included as predictors: total IFS score, FI score, and group. For all analysis, the statistical significance level was set at p < 0.05, and effect sizes were reported.
Additionally, we subdivided the AOs group into two subgroups depending on the degree of harm committed in criminal act. The first group included offenses in which direct harm toward others was committed (n = 12). Offenses within this group included homicide attempt and homicide. The other group included no lethal offenses (theft, illegal carry of weapon, and extortion) or sexual violence (n = 7). Executive functioning, FI, and envy and Schadenfreude ratings were compared with Mann-Whitney U tests.
We also performed Cook’s distance test as means for detecting outliers. We identified three outliers: two AOs and one control; these subjects were excluded from all analysis. We highlight that the main analysis reported here includes the whole sample, whereas results from analysis excluding the outliers can be found exclusively in Supplementary Data (S2).
MRI preprocessing
All image analysis steps were conducted in accordance with VBM12 in the Statistical Parametric Mapping 12 package (SPM12) running under MATLAB 2012. For the pre-processing stage and following previous procedures (de la Fuente et al., 2019; Farokhian, Beheshti, Sone, & Matsuda, 2017), all images were normalized using an affine transformation followed by nonlinear registration, corrected for bias field homogeneities, and then segmented into gray matter, white matter, and cerebrospinal fluid components using the DARTEL algorithm. All segmented, modulated, and normalized gray matter images were smoothed using 8-mm, full-width, half-maximum Gaussian smoothing. Volume changes between images were controlled with Jacobian for the subsequent statistical analyses (see section below).
MRI–behavior associations (Voxel-based morphometry, VBM)
Following previous procedures for the analysis of the neural correlates of envy and Schadenfreude (Santamaría-García et al., 2017, 2019), we conducted multiple regression models. A regression model was conducted for each of the emotions. We conducted a two-step analysis; the first step consisted of a grouped set analysis (both the initial and AOs and NOs groups together), and the second step analyzed AOs separately. Grouped analyses served to increase statistical power through the creation of a larger sample (Irish, Piguet, Hodges, & Hornberger, 2014; O’Callaghan et al., 2016; Sedeño et al., 2016; Sollberger et al., 2009). We acknowledge that this step may be limited as samples differ in multiple characteristics unexplained by brain areas. With that in mind, we conducted a second-step model exclusively in the study group (AOs), which allowed us to explore which areas are particularly involved in determining cognitive and emotional phenomena (García-Cordero et al., 2016; Melloni et al., 2016; Rorden & Karnath, 2004; Shahid et al., 2017). Furthermore, this proceeding has been previously used in studies on the anatomical correlates of social cognition (Melloni et al., 2016; Rorden & Karnath, 2004; Sedeño et al., 2016; Shahid et al., 2017). Following previous procedures (Santamaría-García et al., 2017), we performed a restrictive analysis using a mask including the basal ganglia, bilateral prefrontal cortex, temporal lobes, inferior and superior parietal lobules, precuneus, ACC, and insula. For the prefrontal cortex, we used the Brodmann atlas and included the following areas: frontal eye field (BA8), dorsolateral and medial prefrontal cortex (BA9), anterior prefrontal cortex (BA10), orbitofrontal cortex (BA11), rostral portion of frontal lobe (BA12), inferior frontal gyrus (pars opercularis; BA44), triangular part of inferior frontal gyrus (BA45); dorsolateral prefrontal cortex (BA46), and orbital part of inferior frontal gyrus (BA47). These areas have been consistently reported to be involved in the processing and regulation of social emotions (Baez, Pino, et al., 2018; Frith, 2001; Moll et al., 2002; Moll et al., 2001). Each group was analyzed separately. Total intracranial volume was included as a noninterest covariate in all analyses. The statistical threshold for the statistical analyses was defined as p < 0.001 (uncorrected extent threshold = 30 voxels). We considered this threshold based on several reasons. First, our approach was restricted to the main areas reported to be involved in the processing and regulation of social emotions, which considerably reduced the number of comparisons performed. Second, this statistical threshold is a standard value used in numerous voxel-wise VBM analyses in healthy and clinical populations (de la Fuente et al., 2019; Donix et al., 2013; Irish et al., 2014; Melloni et al., 2016; Rabinovici et al., 2008; Santamaría-García et al., 2019; Sedeño et al., 2017). Third, the thresholding criteria is based on current recommendations of the Organization for Human Brain Mapping (OHBM) (Nichols et al., 2017; Poldrack et al., 2017).
Results
An initial set of analysis including 19 AOs and 20 controls was reported. Then, a second set of analysis excluding three outliers (two AOs and one control; leaving a total of 17 AOs and 19 controls) was performed. Results from the latter analysis were similar to our previous findings (see Supplementary Data S2). We then performed a complementary analysis with a second group of AOs (n = 16) and the original control group (n = 20).
Executive functions and fluid intelligence
Compared with the controls, AOs had significantly lower IFS total scores (F(1, 37) = 4.90, p < 0.05, ηp2 = 0.11). The AOs group also exhibited a significantly lower performance than controls in the motor inhibitory control (F(1,37) = 4.17, p < 0.05, ηp2 = 0.01) and the verbal inhibitory control (F(1,37) = 22.01, p < 0.001, ηp2 = 0.37) subscales. Additionally AOs performed better than NOs in the IFS conflicting instructions (F(1,37) = 4.50, p < 0.05, ηp2 = 0.10) subscale. However, the results showed no significant differences between groups in FI (F(1,37) = 0.50, p = 0.48, ηp2 = 0.01 (Table 1). Results after excluding three outliers (2 AOs and 1 control) revealed that AOs continue to have lower IFS total score as well as lower scores in the motor inhibitory control, backward digit span, and verbal inhibitory control subscales. Details on these results are reported in the Supplementary Data (S2).
Regarding our analysis on the subdivided AOs group, there were no differences in EFs between the direct harm (mdn = 19) and the nonlethal/sexual violence (mdn = 19) groups (U = 41; p = 0.96). There also were no differences in FI between the direct harm (mdn = 19) and the nonlethal/sexual violence (mdn = 21) groups (U = 36.5; p = 0.67).
Social emotions
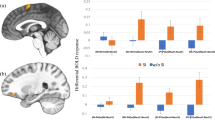
There were group differences, with AOs having significantly lower levels of both envy (F(1,37) = 23.13, p < 0.001, ηp2 = 0.38) and Schadenfreude (F(1, 37) = 14.42, p < 0.001, ηp2 = 0.28) than controls. We performed post-hoc power analysis for these significant effects. For envy, analysis revealed a power of 99.7%, including the following parameters for AOs (n = 19, M = 6.66, SD = 0.03) and NOs (n = 20, M = 7.95, SD = 0.59). Schadenfreude analysis revealed a power of 96.1%, with the following parameters: for AOS (n = 19, M = 5.99, SD = 2.02) and NOs (n = 20, M = 7.87, SD = 0.89). For all analyses, alpha values were set at 0.05.
A further model adding EFs, age, education, and social status as a covariates showed that group effects remained significant for Schadenfreude (F(1, 33) = 12.00, p = 0.001, ηp2 = 0.26) and envy (F(1,33) = 14.46, p < 0.001, ηp2 = 0.30). Regarding the neutral situations, there were no significant differences between groups (F(1,33) = 2.30, p = 0.13, ηp2 = 0.05).
For the analysis on the subdivided AOs group, there were no differences in envy scores between the direct harm (mdn = 7.13) and the nonlethal/sexual violence (mdn = 6.93) groups (U = 37; p = 0.70). Similarly, for Schadenfreude, there were no differences between the direct harm (mdn = 5.87) and the nonlethal/sexual violence (mdn = 6.60) groups (U = 37; p = 0.71).
Relationship between social emotions, EFs, and FI
We estimated two models in which Schadenfreude and envy ratings were considered dependent variables. Total IFS scores, FI scores, and group were included as predictors in both models. As group was included as a predictor, reported beta values corresponds to analysis performed on both groups together. A first model including envy ratings as the dependent variable (F(3, 38) = 10.25, p < 0.001, R2 = 0.48) showed that group (beta = −0.48, p < 0.001) and EFs (beta = 0.38, p = 0.02) were significant predictors of envy (Fig. 2). Similarly, a second model, including Schadenfreude ratings as the dependent variable (F(3, 38) = 7.80, p < 0.001, R2 = 0.41) revealed that group (beta = −0.45, p = 0.01) and EFs (beta = 0.32, p < 0.05) were significant predictors of Schadenfreude (Fig. 2).
Additionally, the correlations between EFs and social emotions showed that, in AOs, envy scores were positively correlated with the IFS total scores (r = 0.44, p = 0.050), as well as the motor inhibitory control (r = 0.46, p = 0.046) and the verbal working memory (r = 0.50, p < 0.05) subscales. Schadenfreude scores were positively correlated with the IFS total scores (r = 0.48, p < 0.05) and the backward digit span subscale (r = 0.44, p < 0.05).
Considering our relatively small sample size, we analyzed a second group of AOs (n = 16) with the original control group. The complementary results replicated previous findings (relative controls, AOs exhibit lower IFS total scores, envy, and Schadenfreude levels). Similarly, envy and Schadenfreude group differences remained significant after controlling for EFs, type of crime, age, education, and social status. Multiple regression analyses showed that EFs and group was significantly associated with both social emotions.
-
3.4.
Brain-behavior associations
Analysis, including both groups (Table 2), revealed positive associations between envy ratings and GM volumes in the following areas: the right precuneus, left superior temporal pole, left inferior temporal gyrus, right superior orbital gyrus, and left rectus gyrus. For Schadenfreude ratings, we found positive associations with the GM volumes in the precuneus (bilaterally), left superior temporal pole, insula, anterior cingulate, right frontal gyrus (superior orbital), and left inferior temporal gyrus.
Results for both groups together. First column shows the type of emotion (variable of interest). Second column shows the regions positively associated to each emotion. Third column shows the cluster size. The following three columns indicate the MNI coordinates for each region.
A similar pattern was observed when analyzing brain-behavior correlations in AOs only (Table 3). Envy ratings were positively associated with GM volumes in the left inferior temporal gyrus, left middle temporal gyrus, left precuneus, left rectus gyrus, and left insula (Fig. 1B). Schadenfreude ratings were positively associated with GM volume in the left inferior temporal gyrus/fusiform gyrus, left IPL, precuneus (bilaterally), left rectus gyrus, left insula and left inferior orbital gyrus (Figure 1C).
Behavioral performance and neural correlates of envy and Schadenfreude. (A) Between-groups comparison in the social-emotions task. The left-most plot shows significantly lower envy scores for AOs compared with NOs. The middle plot shows that AOs exhibited significantly lower levels of Schadenfreude compared with NOs. The right-most plot shows no significant differences in the neutral situations between groups. (B) Brain regions positively associated with reduced envy in AOs (p < 0.001, uncorrected). These regions include the temporal, parietal, and frontal regions. (C) Brain regions associated with reduced Schadenfreude in AOs (p < 0.001, uncorrected). Regions include frontoparietal and temporal regions
Replication of behavioral results
Considering our relatively small sample size, and in order add a replication study, we recruited and analyzed the data of a second group of AOs (n = 16) from the same reeducation and social reinsertion center for young male offenders where the original sample was recruited. Offenses from these subjects also ranged from theft (qualified or aggravated, 37.5%), homicide attempt (6.25%), homicide (25%), and illegal carry of a weapon (31.25%). This second group was also matched in terms of sex and age (F (1, 34) = 0.32, p = 0.85, ηp2 = 0.001) with the original control group.
Executive functions and fluid intelligence
Regarding EFs and FI, this second group of AOs had significantly lower IFS total scores (F (1,34) = 18.27, p < 0.001, ηp2 = 0.35), backward digit span (F(1,34) = 4.98, p < 0.05, ηp2 = 0.12), and verbal inhibitory control (F (1,34) = 24.36, p < 0.001, ηp2 = 0.41) scores.
Social emotions
As for social emotions, AOs exhibited significantly lower levels of both envy (F(1,34) = 25.40, p < 0.001, ηp2 = 0.42) and Schadenfreude (F(1,34) = 15.10, p < 0.001, ηp2 = 0.30) than the controls. The group effects for envy (F(1,30) = 8.75, p < 0.01, ηp2 = 0.02) and Schadenfreude (F(1,30) = 5.64, p < 0.05, ηp2 = 0.15) persisted after controlling for EFs, age, education, and social status. Regarding the neutral situations, there were no significant differences between groups (F(1,37) = 0.001, p = 0.96).
Relationship between social emotions, EFs, and FI
Following the initial analysis protocol, we estimated two models in which Schadenfreude and envy ratings were considered dependent variables. Total IFS scores, FI scores, and group were included as predictors in both models. The first model including envy ratings as the dependent variable (F(3, 34) = 7.58, p = 0.001, R2 = 0.42) showed that group (beta = −0.33, p = 0.05) and EFs (beta = 0.38, p = 0.02) were significant predictors of envy. Similarly, a second model including Schadenfreude ratings as the dependent variable (F(3, 34) = 15.35, p < 0.001, R2 = 0.55) revealed that EFs (beta = 0.57, p < 0.01) was a significant predictor of Schadenfreude. Group showed a tendency for significance (beta = −0.28, p = 0.05)
Discussion
To our knowledge, this study is the first to assess social emotions, their neural correlates, and their relationship with EFs and FI in AOs. The results showed that AOs exhibited significantly lower envy and Schadenfreude levels. These differences persisted after including EFs, age, education, and social status as covariates. This procedure may account for potential extraneous effects that rise in EFs in individuals within this age range. Additionally, we found that in AOs, envy and Schadenfreude were associated with GM volumes in regions subserving mentalizing abilities (IPL, precuneus) and socioemotional processing (inferior and middle temporal regions), as well as key hubs of the executive frontoparietal network (IPL, orbital, and rectus gyri). These results shed light on the structural basis of the brain and cognitive processes underlying social emotions in AOs.
Consistent with a large body of research supporting the idea of the abnormal processing of basic emotions in AOs (Bowen et al., 2014; Fairchild et al., 2009; Gonzalez-Gadea et al., 2014; Jones et al., 2007; Marsh & Blair, 2008; McCown et al., 1986; Sato et al., 2009), our findings suggest that AOs exhibit an abnormal under experience of envy and Schadenfreude as measured with our social emotion task. The observed under experience of envy and Schadenfreude in AOs may be explained by their marked decline on the prerequisites for the evocation of these social emotions (EFs and ToM) (Dolan & Fullam, 2004; Mariano et al., 2017; Möller et al., 2014; Richell et al., 2003; Shamay-Tsoory et al., 2010). Furthermore, research on antisocial personality disorder shows that poor ToM performance in these populations is associated with emotional processing and dysregulation (Bateman et al., 2013; Dolan & Fullam, 2004; Shamay-Tsoory et al., 2010). Importantly, these difficulties in understanding others (exhibited in AOs and antisocial personality disorder) may hinder individual’s feelings of pleasure/displeasure for others fortune/misfortunes. This pattern of envy and Schadenfreude experience may initially appear counterintuitive, because these emotions are considered counter-empathic. However, there is no evidence of such association. On the contrary, there is evidence on prosocial functions of envy and Schadenfreude (Gómez-Carvajal et al., 2020; Shamay-Tsoory et al., 2014; Smith et al., 1996). Because we did not include ToM and empathy measures, futures studies should investigate the relationship between these two domains and social emotions in AOs. Interestingly, similar abnormalities (under experience of social emotions) have been found in clinical populations with emotional and mentalizing issues (Baez, Pino, et al., Baez, Pino, et al., 2018b; Baez, Santamaría-García, Garcia-Cordero, et al., 2016a; Baez, Santamaría-García, Orozco, et al., 2016b).
The present study also showed evidence of lower EFs performance in AOs compared with controls. This finding is consistent with previous studies showing poor EFs performance in AOs (Burton et al., 2016; Carroll et al., 2006; Gonzalez-Gadea et al., 2014; Koolhof et al., 2007; Santamaría-García et al., 2019; Syngelaki et al., 2009; Zou et al., 2013). Furthermore, as previously reported (Santamaría-García et al., 2017), EFs were significantly associated with both envy and Schadenfreude levels. In line with these findings, there were positive correlations between both social emotions and the IFS total scores, and performance in inhibitory control and working memory. The latter two are known to be core EFs (Miyake et al., 2000). These results are consistent with previous reports showing that executive dysfunction in violent and nonviolent offenders is associated with impaired emotional processing (Hoaken et al., 2007). These findings also converge with evidence in clinical populations showing that affected EFs may negatively impact emotion recognition (Baez, Couto, et al., 2014a; Henry et al., 2006; Kohler et al., 2000). Moreover, neurotypical individuals with emotion recognition difficulties report difficulty in executive functioning in daily life (Suchy, 2009). Thus, our findings and previous evidence suggest that EFs may be an important factor associated with diminished social emotional experiences in this group. However, considering that the IFS was developed as a screening tool, we acknowledge that using it as a standalone measure for EFs represents a limitation of our study. Future research should use extensive batteries when assessing the specific contribution of EFs to social emotions in AOs.
Structural brain correlates of social emotions in AOs showed similar associations for envy and Schadenfreude. Specifically, we found that experiencing these emotions was positively associated with GM volumes in the inferior and middle temporal gyri, precuneus, IPL, and rectus and inferior orbital gyri. These aforementioned regions are associated with envy and Schadenfreude (Baez, García, & Santamaría-García, 2017a; Baez, Herrera, et al., 2018a; Santamaría-García et al., 2017; Takahashi et al., 2009). In particular, the temporal lobe plays a crucial role in the understanding of social concepts and rules that trigger envy and Schadenfreude (Baez, Pino, et al., 2018b). Moreover, research on clinical populations (Couto et al., 2013; Melloni et al., 2016; O’Callaghan et al., 2016) has shown that damage to frontotemporal networks results in a failure to integrate the self-perspective with that of others, a main component needed to experience social emotions (Baez, Pino, et al., 2018b). In addition, the parietal lobe, including the IPL and precuneus, is a key hub of the mentalizing network, necessarily recruited when experiencing envy and Schadenfreude (Baez, Pino, et al., 2018b; Shamay-Tsoory, 2011). Parietal and frontal regions also are involved in other social cognition domains, such as moral cognition (Moll et al., 2005), especially emotions favoring social adherence (Moll et al., 2005). For instance, a negative outcome following a moral code breach may trigger feelings of pleasure (Schadenfreude), while a positive outcome that is not morally deserved may trigger feelings of displeasure (envy). The experience of pleasure/displeasure acts as a motivator for seeking justice (Dvash et al., 2010; Feather & Sherman, 2002; Haidt, 2003; Jankowski & Takahashi, 2014; Najle, 2015; Smith & Kim, 2007; van Dijk & Ouwerkerk, 2014; Yoder & Decety, 2014).
Frontal and parietal areas are also part of a widely distributed frontoparietal network involved in EFs (Carlesimo et al., 2001; Diwadkar et al., 2000; Kondo et al., 2004; Li et al., 2004; Mazoyer et al., 2001; McEvoy et al., 2001; Sauseng et al., 2004; Sauseng et al., 2005). Thus, consistent with the reported link between EFs and reduced social emotions in AOs, we found a significant association between frontal (gyrus rectus, orbitofrontal cortex) and parietal (precuneus, IPL) regions that have been previously associated with executive functioning.
When combining both groups, we found a positive association between both social emotions and GM volumes in the ACC. The ACC has been shown to be involved in socioemotional processes (Allman et al., 2001; Bechara, 2004; Bush et al., 2000; Etkin et al., 2011; Lane et al., 1998). Specifically, it has been argued that it serves as an integration center for inputs coming from emotional and cognitive networks (Allman et al., 2001; Bush et al., 2000). In fact, the ACC is activated in response to envy triggering stimuli but also predicts neural mechanisms involved in experiencing Schadenfreude (Takahashi et al., 2009). Thus, the ACC may be a region that allows the necessary empathic and mentalizing abilities as well as other social cognitive aspects of experience of envy and Schadenfreude.
Some limitations of our study should be acknowledged. The first concerns our relatively small sample size. We acknowledge that small sample sizes may affect interpretation of results. In order to overcome this possible shortcoming, we performed a replications study where we recruited an additional sample of AOs (n = 16) and compared them with the original control group. Results of these complementary comparisons replicated the original ones. This strategy contributed to the validity of our results and allowed us to reduce potential sample size limitations. However, further studies on neural correlates of social emotions in AOs should include larger sample sizes. A second limitation pertains to the heterogeneous nature of criminal behaviour. Research shows that the nature of the criminal/antisocial behaviour is related to cognitive abilities and EFs (Burt, 2012; Loughran et al., 2012; Piquero & White, 2003; Sigurdsson, Gudjonsson, & Peersen, 2001). Given that our sample contained individuals with a range of criminal offenses, it might be the case that differences in type of crime might play a role in the observed group differences. To address this issue, we divided the AOs group into those who committed direct harm (homicide attempt and homicide) and those who did not commit lethal harm on others (theft, illegal carry of weapon, extortion, and sexual abuse). We did not find any group effect for our dependent variables. However, considering our sample size, future studies should further explore the potential differences between AO subgroups. Moreover, future studies may benefit from an exhaustive characterization of the type of offenses and the level of harm caused to the victim. A second limitation concerns the potential influence of personality factors on the observed differences. Antisocial personality disorder is closely related to impaired social cognition, poor mentalizing abilities and increased criminal/behavior (Newbury-Helps et al., 2017). Failing to include personality measures posits a limitation of the present study. Future studies should include personality measures. A third limitation could relate to motivation factors as we did not include measures of the participants’ motivation to perform the tests. Considering the assessed population, this may pose a limitation in our study, because nonmotivated and bored individuals may not perform appropriately. However, it is important to consider that all AOs voluntarily chose between performing their daily life activities within the Centre or participating in our study. Despite the subject’s voluntary choice to take part in our study, future research on social cognition in AOs should explore the potential effect of lack of motivation. Another potential weakness lies within the anatomical analyses. Our VBM analysis relied on a restrictive mask, a process that could have filtered out structural information. Thus, future studies could benefit from using a whole brain analysis in VBM. However, we chose our regions of interest (ROI) based on our research question and evidence on brain regions involved in envy and Schadenfreude. The use of ROI analysis has been previously used on social emotion structural neuroimaging studies (Santamaría-García et al., 2017). Additionally, we acknowledge a further limitation pertaining the lack of corrections for multiple analyses for neuroimaging results. However, the decision to report uncorrected results was made considering our sample size. The same threshold has been previously employed in several VBM studies (de la Fuente et al., 2019; Donix et al., 2013; Irish et al., 2014; Melloni et al., 2016; Rabinovici et al., 2008; Santamaría-García et al., 2019; Sedeño et al., 2017). Moreover, mask-restricted analysis focuses on a small number of areas, thereby reducing the multiple comparison issues inherent to multi-voxel analysis.
Lastly, the social emotion task used; that the situations triggering envy/Schadenfreude may lack from a more ecological picture of the target character for each situation. For instance, a given situation triggering either envy or Schadenfreude could happen to a target character that has acted in an anti/prosocial way to a second character. This extra variable may yield interesting and more ecological findings. Thus, future studies could benefit from a more complex dynamics involving the target characters.
In summary, social emotions such as envy and Schadenfreude are complex phenomena that tap into distinct but overlapping networks responsible for mentalizing, emotional processing, and EFs. Given the importance of envy and Schadenfreude as regulators of social dynamics (Dvash et al., 2010; Feather & Sherman, 2002; Haidt, 2003; Jankowski & Takahashi, 2014; Najle, 2015; Smith & Kim, 2007; van Dijk & Ouwerkerk, 2014; Yoder & Decety, 2014), abnormalities in experiencing such emotions may be related to poor adaptation to society or the lack of prosocial behavior observed in AOs. The findings presented here may build on a theoretical understanding of the intricacies of social emotions and the social brain. Additionally, our results may have translational implications for the prediction and treatment of social adaptation. In terms of predictability, the domains of social cognition and EFs may serve as an index for future social adaptation and offending behavior. These results are in line with the emotional structural account. This approach suggests that emotions raise in response to power-status outcomes of social interactions (Fine & Kemper, 1981; Goodwin et al., 2013; Kemper, 1978, 1991; Kemper & Lazarus, 1992). More specifically, changes in perception of self-status and/or power with respect to others give rise to a variety of emotions. For instance, feelings of envy could rise when an individual witnesses an unlawful increase in other’s status/power of another (Smith, 1991). Similarly, experience of Schadenfreude can be triggered by observing a negative outcome on someone with a desired level of status or power (Smith et al., 2009). These explanations fit perfectly with our findings, because the social comparison status and power dimensions between self and others requires of EFs and ToM, which are impaired in AOs. Therefore, this makes it difficult to assess others states in the so-called dimensions (status or power). Such difficulties may result in decreased experience of envy and Schadenfreude. Additionally, interventions based on social cognition and EFs may prove to be effective in the rehabilitation of AOs, thus increasing their chances of social adaptation. Further research is required to investigate the impact of EFs training on the ability to experience envy and Schadenfreude in AOs. Additionally, future research should assess the specific relationship between social emotions, social adaptation, and offending behavior in AOs. Such studies may have important implications for subsequent adaptation and reintegration to society.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E., Hof, P., & Hixon, F. P. (2001). The anterior cingulate cortex: The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences https://doi.org/10.1111/j.1749-6632.2001.tb03476.x
Baez, S., Couto, B., Torralva, T., Sposato, L. A., Huepe, D., Montañes, P., … Ibanez, A. (2014a). Comparing moral judgments of patients with frontotemporal dementia and frontal stroke. JAMA Neurology https://doi.org/10.1001/jamaneurol.2014.347
Baez, S., García, A. M., & Santamaría-García, H. (2017a). Moral cognition and moral emotions. In Neuroscience and Social Science: The Missing Link. https://doi.org/10.1007/978-3-319-68421-5_8
Baez, S., Herrera, E., García, A. M., Manes, F., Young, L., & Ibáñez, A. (2017b). Outcome-oriented moral evaluation in terrorists. Nature Human Behaviour https://doi.org/10.1038/s41562-017-0118
Baez, S., Ibanez, A., Gleichgerrcht, E., Perez, A., Roca, M., Manes, F., & Torralva, T. (2014b). The utility of IFS (INECO Frontal Screening) for the detection of executive dysfunction in adults with bipolar disorder and ADHD. Psychiatry Research https://doi.org/10.1016/j.psychres.2014.01.020
Baez, S., Pino, M., Berrío, M., Santamaría-García, H., Sedeño, L., García, A. M., … Ibáñez, A. (2018b). Corticostriatal signatures of schadenfreude: Evidence from Huntington’s disease. Journal of Neurology, Neurosurgery and Psychiatry https://doi.org/10.1136/jnnp-2017-316055
Baez, S., Santamaría-García, H., Orozco, J., Fittipaldi, S., García, A. M., Pino, M., & Ibáñez, A. (2016b). Your misery is no longer my pleasure: Reduced schadenfreude in Huntington’s disease families. Cortex https://doi.org/10.1016/j.cortex.2016.07.009
Bardeen, J. R., Stevens, E. N., Murdock, K. W., & Christine Lovejoy, M. (2013). A preliminary investigation of sex differences in associations between emotion regulation difficulties and higher-order cognitive abilities. Personality and Individual Differences https://doi.org/10.1016/j.paid.2013.02.003
Bateman, A., Bolton, R., & Fonagy, P. (2013). Antisocial Personality Disorder: A Mentalizing Framework. FOCUS. https://doi.org/10.1176/appi.focus.11.2.178
Bechara, A. (2004). Disturbances of Emotion Regulation After Focal Brain Lesions. International Review of Neurobiology https://doi.org/10.1016/S0074-7742(04)62006-X
Bergeron, T. K., & Valliant, P. M. (2001). Executive function and personality in adolescent and adult offenders vs. non-offenders. Journal of Offender Rehabilitation. https://doi.org/10.1300/J076v33n03_02
Berkman, E. T., Cunningham, W. A., & Lieberman, M. D. (2014). Research Methods in Social and Affective Neuroscience. In Handbook of Research Methods in Social and Personality Psychology https://doi.org/10.1017/cbo9780511996481.011
Blakemore, S. J., & Choudhury, S. (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry and Allied Disciplines https://doi.org/10.1111/j.1469-7610.2006.01611.x
Bowen, K. L., Morgan, J. E., Moore, S. C., & Van Goozen, S. H. M. (2014). Young offenders’ emotion recognition dysfunction across emotion intensities: Explaining variation using psychopathic traits, conduct disorder and offense severity. Journal of Psychopathology and Behavioral Assessment https://doi.org/10.1007/s10862-013-9368-z
Burt, S. A. (2012). How do we optimally conceptualize the heterogeneity within antisocial behavior? An argument for aggressive versus non-aggressive behavioral dimensions Clinical Psychology Review https://doi.org/10.1016/j.cpr.2012.02.006
Burton, D., Demuynck, S., & Yoder, J. R. (2016). Executive Dysfunction Predicts Delinquency But Not Characteristics of Sexual Aggression Among Adolescent Sexual Offenders. Sexual Abuse: Journal of Research and Treatment. https://doi.org/10.1177/1079063214556357
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences https://doi.org/10.1016/S1364-6613(00)01483-2
Cai, H., & Liu, C. (2004). Anterior Cingulate Cortex and Executive Function. Advances in Psychological Science, 12(05), 643–650.
Carlesimo, G. A., Perri, R., Turriziani, P., Tomaiuolo, F., & Caltagirone, C. (2001). Remembering what but not where: Independence of spatial and visual working memory in the human brain. Cortex https://doi.org/10.1016/S0010-9452(08)70591-4
Carlson, S. M., & Moses, L. J. (2001). Individual Differences in Inhibitory Control and Children’s Theory of Mind. Child Development https://doi.org/10.1111/1467-8624.00333
Carlson, S. M., Moses, L. J., & Breton, C. (2002). How Specific is the Relation between Executive Function and Theory of Mind? Contributions of Inhibitory Control and Working Memory Infant and Child Development https://doi.org/10.1002/icd.298
Carroll, A., Hemingway, F., Bower, J., Ashman, A., Houghton, S., & Durkin, K. (2006). Impulsivity in juvenile delinquency: Differences among early-onset, late-onset, and non-offenders. Journal of Youth and Adolescence https://doi.org/10.1007/s10964-006-9053-6
Chester, D. S., Powell, C. A. J., Smith, R. H., Joseph, J. E., Kedia, G., Combs, D. J. Y., & DeWall, C. N. (2013). Justice for the average Joe: The role of envy and the mentalizing network in the deservingness of others’ misfortunes. Social Neuroscience. https://doi.org/10.1080/17470919.2013.846278
Couto, B., Manes, F., Montañés, P., Matallana, D., Reyes, P., Velasquez, M., … Ibáñez, A. (2013). Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Frontiers in Human Neuroscience https://doi.org/10.3389/fnhum.2013.00467
de la Fuente, A., Sedeño, L., Vignaga, S. S., Ellmann, C., Sonzogni, S., Belluscio, L., … Ibañez, A. (2019). Multimodal neurocognitive markers of interoceptive tuning in smoked cocaine. Neuropsychopharmacology https://doi.org/10.1038/s41386-019-0370-3
De Stasio, S., Fiorilli, C., & Di Chiacchio, C. (2014). Effects of verbal ability and fluid intelligence on children’s emotion understanding. International Journal of Psychology https://doi.org/10.1002/ijop.12032
Diwadkar, V. A., Carpenter, P. A., & Just, M. A. (2000). Collaborative activity between parietal and dorso-lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. NeuroImage https://doi.org/10.1006/nimg.2000.0586
Dodonova, Y. A., & Dodonov, Y. S. (2012). Speed of emotional information processing and emotional intelligence. International Journal of Psychology https://doi.org/10.1080/00207594.2012.656131
Dolan, M., & Fullam, R. (2004). Theory of mind and mentalizing ability in antisocial personality disorders with and without psychopathy. Psychological Medicine https://doi.org/10.1017/S0033291704002028
Donix, M., Jurjanz, L., Meyer, S., Amanatidis, E. C., Baeumler, D., Huebner, T., … Holthoff, V. A. (2013). Functional imaging during recognition of personally familiar faces and places in Alzheimer’s disease. Archives of Clinical Neuropsychology https://doi.org/10.1093/arclin/acs093
Donnellan, M. B., Ge, X., & Wenk, E. (2000). Cognitive abilities in adolescent-limited and life-course-persistent criminal offenders. Journal of Abnormal Psychology https://doi.org/10.1037/0021-843X.109.3.396
Dvash, J., Gilam, G., Ben-Ze’ev, A., Hendler, T., & Shamay-Tsoory, S. G. (2010). The envious brain: The neural basis of social comparison. Human Brain Mapping https://doi.org/10.1002/hbm.20972
Ellis, H. D., & Gunter, H. L. (1999). Asperger syndrome: A simple matter of white matter? Trends in Cognitive Sciences, 3, 192–200.
Etkin, A., Egner, T., & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. https://doi.org/10.1016/j.tics.2010.11.004
Fairchild, G., Van Goozen, S. H. M., Calder, A. J., Stollery, S. J., & Goodyer, I. M. (2009). Deficits in facial expression recognition in male adolescents with early-onset or adolescence-onset conduct disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines https://doi.org/10.1111/j.1469-7610.2008.02020.x
Farokhian, F., Beheshti, I., Sone, D., & Matsuda, H. (2017). Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Frontiers in Neurology, 8(AUG). https://doi.org/10.3389/fneur.2017.00428
Feather, N. T., & Sherman, R. (2002). Envy, resentment, schadenfreude, and sympathy: Reactions to deserved and undeserved achievement and subsequent failure. Personality and Social Psychology Bulletin https://doi.org/10.1177/014616720202800708
Ferner, J., & Lang, B. (1999). Development of theory of mind and executive control. Trends in Cognitive Sciences https://doi.org/10.1016/S1364-6613(99)01362-5
Fine, G. A., & Kemper, T. D. (1981). A Social Interactional Theory of Emotions. Social Forces https://doi.org/10.2307/2578010
Frith. (2001). Mind blindness and the brain in autism. Neuron https://doi.org/10.1016/S0896-6273(01)00552-9
García-Cordero, I., Sedeño, L., de la Fuente, L., Slachevsky, A., Forno, G., Klein, F., … Ibañez, A. (2016). Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society B: Biological Sciences https://doi.org/10.1098/rstb.2016.0006
Gómez-Carvajal, A. M., Santamaría-García, H., García, A. M., Valderrama, M., Mejia, J., Santamaría-García, J., … Baez, S. (2020). The unique social sense of puerperium: Increased empathy and Schadenfreude in parents of newborns. Scientific Reports https://doi.org/10.1038/s41598-020-62622-7
Gonzalez-Gadea, M. L., Herrera, E., Parra, M., Mendez, P. G., Baez, S., Manes, F., & Ibanez, A. (2014). Emotion recognition and cognitive empathy deficits in adolescent offenders revealed by context-sensitive tasks. Frontiers in Human Neuroscience https://doi.org/10.3389/fnhum.2014.00850
Goodwin, J., Jasper, J. M., & Polletta, F. (2013). Passionate Politics. In Passionate Politics https://doi.org/10.7208/chicago/9780226304007.001.0001
Gray, J. R., Chabris, C. F., & Braver, T. S. (2003). Neural mechanisms of general fluid intelligence. Nature Neuroscience https://doi.org/10.1038/nn1014
Haidt, J. (2003). The moral emotions. Handbook of Affective Sciences, 11(2003), 852–870.
Henry, J. D., Phillips, L. H., Crawford, J. R., Ietswaart, M., & Summers, F. (2006). Theory of mind following traumatic brain injury: The role of emotion recognition and executive dysfunction. Neuropsychologia https://doi.org/10.1016/j.neuropsychologia.2006.03.020
Herrero, Ó., Escorial, S., & Colom, R. (2019). Rapists and child abusers share low levels in executive updating, but do not in fluid reasoning. European Journal of Psychology Applied to Legal Context https://doi.org/10.5093/ejpalc2018a10
Hoaken, P. N. S., Allaby, D. B., & Earle, J. (2007). Executive cognitive functioning and the recognition of facial expressions of emotion in incarcerated violent offenders, non-violent offenders, and controls. Aggressive Behavior https://doi.org/10.1002/ab.20194
Holland, T., Clare, I. C., & Mukhopadhyay, T. (2002). Prevalence of criminal offending by men and women with intellectual disability and the characteristics of offenders: implications for research and service development. Journal of Intellectual Disability Research : JIDR. https://doi.org/10.1046/j.1365-2788.2002.00001.x
Hubble, K., Bowen, K. L., Moore, S. C., & Van Goozen, S. H. M. (2015). Improving negative emotion recognition in young offenders reduces subsequent crime. PLoS ONE https://doi.org/10.1371/journal.pone.0132035
Ibáñez, A., Petroni, A., Urquina, H., Torrente, F., Torralva, T., Hurtado, E., … Manes, F. (2011). Cortical deficits of emotional face processing in adults with ADHD: Its relation to social cognition and executive function. Social Neuroscience. https://doi.org/10.1080/17470919.2011.620769
Irish, M., Piguet, O., Hodges, J. R., & Hornberger, M. (2014). Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and alzheimer’s disease. Human Brain Mapping https://doi.org/10.1002/hbm.22263
Jankowski, K. F., & Takahashi, H. (2014). Cognitive neuroscience of social emotions and implications for psychopathology: Examining embarrassment, guilt, envy, and schadenfreude. Psychiatry and Clinical Neurosciences https://doi.org/10.1111/pcn.12182
John, & Raven, J. (2003). Raven Progressive Matrices. In Handbook of Nonverbal Assessment https://doi.org/10.1007/978-1-4615-0153-4_11
Jones, A. P., Forster, A. S., & Skuse, D. (2007). What do you think you’re looking at? Investigating social cognition in young offenders Criminal Behaviour and Mental Health https://doi.org/10.1002/cbm.641
Jusyte, A., & Schönenberg, M. (2017). Impaired social cognition in violent offenders: perceptual deficit or cognitive bias? European Archives of Psychiatry and Clinical Neuroscience https://doi.org/10.1007/s00406-016-0727-0
Kelly, T., Richardson, G., Hunter, R., & Knapp, M. (2002). Attention and executive function deficits in adolescent sex offenders. Child Neuropsychology https://doi.org/10.1076/chin.8.2.138.8722
Kemper. (1978). Toward a Sociology of Emotions : Some Problems and Some Solutions. The American Sociologist.
Kemper. (1991). Predicting Emotions from Social Relations. Social Psychology Quarterly https://doi.org/10.2307/2786845
Kemper, & Lazarus. (1992). Emotion and Adaptation. Contemporary Sociology https://doi.org/10.2307/2075902
Kohler, C. G., Bilker, W., Hagendoorn, M., Gur, R. E., & Gur, R. C. (2000). Emotion recognition deficit in schizophrenia: Association with symptomatology and cognition. Biological Psychiatry https://doi.org/10.1016/S0006-3223(00)00847-7
Kondo, H., Morishita, M., Osaka, N., Osaka, M., Fukuyama, H., & Shibasaki, H. (2004). Functional roles of the cingulo-frontal network in performance on working memory. NeuroImage https://doi.org/10.1016/j.neuroimage.2003.09.046
Koolhof, R., Loeber, R., Wei, E. H., Pardini, D., & D’Escury, A. C. (2007). Inhibition deficits of serious delinquent boys of low intelligence. Criminal Behaviour and Mental Health https://doi.org/10.1002/cbm.661
Kuin, N. C., Masthoff, E. D. M., Nunnink, V. N., Munafò, M. R., & Penton-Voak, I. S. (2019). Changing Perception: A Randomized Controlled Trial of Emotion Recognition Training to Reduce Anger and Aggression in Violent Offenders. Psychology of Violence. https://doi.org/10.1037/vio0000254
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., & Eickhoff, S. B. (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure & Function https://doi.org/10.1007/s00429-010-0255-z
Lane, R. D., Reiman, E. M., Axelrod, B., Yun, L. S., Holmes, A., & Schwartz, G. E. (1998). Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience https://doi.org/10.1162/089892998562924
Lang, B., & Perner, J. (2002). Understanding of intention and false belief and the development of self-control. British Journal of Developmental Psychology https://doi.org/10.1348/026151002166325
Leach, C. W., Spears, R., Branscombe, N. R., & Doosje, B. (2003). Malicious Pleasure: Schadenfreude at the Suffering of Another Group. Journal of Personality and Social Psychology. https://doi.org/10.1037/0022-3514.84.5.932
Leshem, R., van Lieshout, P. H. H. M., Ben-David, S., & Ben-David, B. M. (2019). Does emotion matter? The role of alexithymia in violent recidivism: A systematic literature review Criminal Behaviour and Mental Health. https://doi.org/10.1002/cbm.2110
Li, Su, Y., Liu, X., Shi, W., & Shi, K. (2017). Prosocial behavior in envy scenarios. Social Behavior and Personality. https://doi.org/10.2224/sbp.6660
Li, Z. H., Sun, X. W., Wang, Z. X., Zhang, X. C., Zhang, D. R., He, S., & Hu, X. P. (2004). Behavioral and functional MRI study of attention shift in human verbal working memory. NeuroImage https://doi.org/10.1016/j.neuroimage.2003.08.043
Loughran, T. A., Piquero, A. R., Fagan, J., & Mulvey, E. P. (2012). Differential deterrence: Studying heterogeneity and changes in perceptual deterrence among serious youthful offenders. Crime and Delinquency https://doi.org/10.1177/0011128709345971
Mariano, M., Pino, M. C., Peretti, S., Valenti, M., & Mazza, M. (2017). Understanding criminal behavior: Empathic impairment in criminal offenders. Social Neuroscience https://doi.org/10.1080/17470919.2016.1179670
Marsh, A. A., & Blair, R. J. R. (2008). Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience and Biobehavioral Reviews https://doi.org/10.1016/j.neubiorev.2007.08.003
Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houdé, O., … Tzourio-Mazoyer, N. (2001). Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin https://doi.org/10.1016/S0361-9230(00)00437-8
McCown, W., Johnson, J., & Austin, S. (1986). Inability of delinquents to recognize facial affects. Journal of Social Behavior & Personality
McEvoy, L. K., Pellouchoud, E., Smith, M. E., & Gevins, A. (2001). Neurophysiological signals of working memory in normal aging. Cognitive Brain Research https://doi.org/10.1016/S0926-6410(01)00009-X
McGrew, K. S. (2009). CHC theory and the human cognitive abilities project: Standing on the shoulders of the giants of psychometric intelligence research. Intelligence https://doi.org/10.1016/j.intell.2008.08.004
Melloni, M., Billeke, P., Baez, S., Hesse, E., De La Fuente, L., Forno, G., … Ibáñez, A. (2016). Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain https://doi.org/10.1093/brain/aww231
Mendez, M. F. (2009). The neurobiology of moral behavior: Review and neuropsychiatric implications. CNS Spectrums https://doi.org/10.1017/S1092852900023853
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cognitive Psychology https://doi.org/10.1006/cogp.1999.0734
Moll, J., De Oliveira-Souza, R., Bramati, I. E., & Grafman, J. (2002). Functional networks in emotional moral and nonmoral social judgments. NeuroImage https://doi.org/10.1006/nimg.2002.1118
Moll, J., Eslinger, P. J., & De Oliveira-Souza, R. (2001). Frontopolar and anterior temporal cortex activation in a moral judgment task: Preliminary functional MRI results in normal subjects. Arquivos de Neuro-Psiquiatria https://doi.org/10.1590/s0004-282x2001000500001
Moll, J., Zahn, R., De Oliveira-Souza, R., Krueger, F., & Grafman, J. (2005). Opinion: The neural basis of human moral cognition. Nature Reviews Neuroscience https://doi.org/10.1038/nrn1768
Najle, M. (2015). Delicious Justice: Schadenfreude Toward Atheists Bound for Hell.
Newbury-Helps, J., Feigenbaum, J., & Fonagy, P. (2017). Offenders with antisocial personality disorder display more impairments in mentalizing. Journal of Personality Disorderss https://doi.org/10.1521/pedi_2016_30_246
Nichols, T. E., Das, S., Eickhoff, S. B., Evans, A. C., Glatard, T., Hanke, M., … Yeo, B. T. T. (2017). Best practices in data analysis and sharing in neuroimaging using MRI. Nature Neuroscience https://doi.org/10.1038/nn.4500
O’Callaghan, C., Bertoux, M., Irish, M., Shine, J. M., Wong, S., Spiliopoulos, L., … Hornberger, M. (2016). Fair play: Social norm compliance failures in behavioural variant frontotemporal dementia. Brain https://doi.org/10.1093/brain/awv315
Ouwerkerk, J. W., van Dijk, W. W., Vonkeman, C. C., & Spears, R. (2018). When we enjoy bad news about other groups: A social identity approach to out-group schadenfreude. Group Processes and Intergroup Relations https://doi.org/10.1177/1368430216663018
Ozonoff, S., Pennington, B. F., & Rogers, S. J. (1991). Executive Function Deficits in High-Functioning Autistic Individuals: Relationship to Theory of Mind. Journal of Child Psychology and Psychiatry. https://doi.org/10.1111/j.1469-7610.1991.tb00351.x
Palmer, E. J., & Hollin, C. R. (1999). Social Competence and Sociomoral Reasoning in Young Offenders. Applied Cognitive Psychology https://doi.org/10.1002/(SICI)1099-0720(199902)13:1<79::AID-ACP613>3.0.CO;2-Q
Paul, S. M. (1986). The advanced raven’s progressive matrices: Normative data for an American university population and an examination of the relationship with spearman’s g. Journal of Experimental Education https://doi.org/10.1080/00220973.1986.10806404
Paulus, F. M., Müller-Pinzler, L., Stolz, D. S., Mayer, A. V., Rademacher, L., & Krach, S. (2018). Laugh or cringe? Common and distinct processes of reward-based schadenfreude and empathy-based fremdscham Neuropsychologia https://doi.org/10.1016/j.neuropsychologia.2017.05.030
Piquero, A. R., & White, N. A. (2003). On the relationship between cognitive abilities and life-course-persistent offending among a sample of African Americans: A longitudinal test of Moffitt’s hypothesis. Journal of Criminal Justice. https://doi.org/10.1016/S0047-2352(03)00046-1
Poldrack, R. A., Baker, C. I., Durnez, J., Gorgolewski, K. J., Matthews, P. M., Munafò, M. R., … Yarkoni, T. (2017). Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience https://doi.org/10.1038/nrn.2016.167
Qi, Y., Nan, W., Cai, H., Wu, H., & Liu, X. (2020). Empathy or schadenfreude? Social value orientation and affective responses to gambling results Personality and Individual Differences. https://doi.org/10.1016/j.paid.2019.109619
Rabinovici, G. D., Seeley, W. W., Kim, E. J., Gorno-Tempini, M. L., Rascovsky, K., Pagliaro, T. A., … Rosen, H. J. (2008). Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. American Journal of Alzheimer’s Disease and Other Dementias https://doi.org/10.1177/1533317507308779
Rademacher, L., Krach, S., Kohls, G., Irmak, A., Gründer, G., & Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage https://doi.org/10.1016/j.neuroimage.2009.10.089
Raven, J. C. (1960). Guide to standard progressive matrices. London: Lewis, HK.
Rey-Mermet, A., Gade, M., Souza, A. S., von Bastian, C. C., & Oberauer, K. (2019). Is executive control related to working memory capacity and fluid intelligence? Journal of Experimental Psychology: General. https://doi.org/10.1037/xge0000593
Roccatagliata, G., & Benassi, E. (1981). Mental impairment and intelligence g Factor: A Psychometric Profile. Journal of Psychology: Interdisciplinary and Applied. https://doi.org/10.1080/00223980.1981.9915261
Rorden, C., & Karnath, H. O. (2004). Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience https://doi.org/10.1038/nrn1521
Santamaría-García, H., Baez, S., Reyes, P., Santamaría-García, J. A., Santacruz-Escudero, J. M., Matallana, D., … Ibáñez, A. (2017). A lesion model of envy and Schadenfreude: Legal, deservingness and moral dimensions as revealed by neurodegeneration. Brain. https://doi.org/10.1093/brain/awx269
Santamaría-García, H., Ibáñez, A., Montaño, S., García, A. M., Patiño-Saenz, M., Idarraga, C., … Baez, S. (2019). Out of context, beyond the face: Neuroanatomical pathways of emotional face-body language integration in adolescent offenders. Frontiers in Behavioral Neuroscience https://doi.org/10.3389/fnbeh.2019.00034
Sato, W., Uono, S., Matsuura, N., & Toichi, M. (2009). Misrecognition of facial expressions in delinquents. Child and Adolescent Psychiatry and Mental Health https://doi.org/10.1186/1753-2000-3-27
Sauseng, P., Klimesch, W., Doppelmayr, M., Hanslmayr, S., Schabus, M., & Gruber, W. R. (2004). Theta coupling in the human electroencephalogram during a working memory task. Neuroscience Letters https://doi.org/10.1016/j.neulet.2003.10.002
Sauseng, P., Klimesch, W., Schabus, M., & Doppelmayr, M. (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology https://doi.org/10.1016/j.ijpsycho.2005.03.018
Sedeño, L., Couto, B., García-Cordero, I., Melloni, M., Baez, S., Sepúlveda, J. P. M., … Ibanez, A. (2016). Brain network organization and social executive performance in frontotemporal dementia. Journal of the International Neuropsychological Society https://doi.org/10.1017/S1355617715000703
Sedeño, L., Piguet, O., Abrevaya, S., Desmaras, H., García-Cordero, I., Baez, S., … Ibanez, A. (2017). Tackling variability: A multicenter study to provide a gold-standard network approach for frontotemporal dementia. Human Brain Mapping https://doi.org/10.1002/hbm.23627
Seeley, W. W., Zhou, J., & Kim, E. J. (2012). Frontotemporal dementia: What can the behavioral variant teach us about human brain organization? Neuroscientist https://doi.org/10.1177/1073858411410354
Shahid, H., Sebastian, R., Schnur, T. T., Hanayik, T., Wright, A., Tippett, D. C., … Hillis, A. E. (2017). Important considerations in lesion-symptom mapping: Illustrations from studies of word comprehension. Human Brain Mapping https://doi.org/10.1002/hbm.23567
Shamay-Tsoory, S. G. (2011). The neural bases for empathy. Neuroscientist https://doi.org/10.1177/1073858410379268
Shamay-Tsoory, S. G., Ahronberg-Kirschenbaum, D., & Bauminger-Zviely, N. (2014). There is no joy like malicious joy: Schadenfreude in young children. PLoS ONE https://doi.org/10.1371/journal.pone.0100233
Shamay-Tsoory, S. G., Harari, H., Aharon-Peretz, J., & Levkovitz, Y. (2010). The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex https://doi.org/10.1016/j.cortex.2009.04.008
Shamosh, N. A., & Gray, J. R. (2007). The relation between fluid intelligence and self-regulatory depletion. Cognition and Emotion https://doi.org/10.1080/02699930701273658
Sierra Sanjurjo, N., Saraniti, A. B., Gleichgerrcht, E., Roca, M., Manes, F., & Torralva, T. (2019). The IFS (INECO Frontal Screening) and level of education: Normative data. Applied Neuropsychology:Adult https://doi.org/10.1080/23279095.2018.1427096
Sigurdsson, Jon F., & Gudjonsson, G. H. (1996). The psychological characteristics of “false confessors”. A study among Icelandic prison inmates and juvenile offenders. Personality and Individual Differences https://doi.org/10.1016/0191-8869(95)00184-0
Sigurdsson, J. F., Gudjonsson, G. H., & Peersen, M. (2001). Differences in the cognitive ability and personality of desisters and re-offenders: A prospective study among young offenders. Psychology, Crime and Law. https://doi.org/10.1080/10683160108401781
Smith, & Kim, S. H. (2007). Comprehending envy. Psychological Bulletin. https://doi.org/10.1037/0033-2909.133.1.46
Smith, Powell, C. A. J., Combs, D. J. Y., & Schurtz, D. R. (2009). Exploring the When and Why of Schadenfreude. Social and Personality Psychology Compass https://doi.org/10.1111/j.1751-9004.2009.00181.x
Smith, R. H. (1991). Envy and the sense of injustice. In The psychology of jealousy and envy.
Smith, Richard H., Turner, T. J., Garonzik, R., Leach, C. W., Urch-Druskat, V., & Weston, C. M. (1996). Envy and Schadenfreude. Personality and Social Psychology Bulletin https://doi.org/10.1177/0146167296222005
Sollberger, M., Stanley, C. M., Wilson, S. M., Gyurak, A., Beckman, V., Growdon, M., … Rankin, K. P. (2009). Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia https://doi.org/10.1016/j.neuropsychologia.2009.06.006
Spreckelmeyer, K. N., Krach, S., Kohls, G., Rademacher, L., Irmak, A., Konrad, K., … Gründer, G. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience https://doi.org/10.1093/scan/nsn051
Staff, R. T, Hogan, M. J., & Whalley, L. J. (2014). Aging trajectories of fluid intelligence in late life: The influence of age, practice and childhood IQ on Raven’s Progressive Matrices. Intelligence https://doi.org/10.1016/j.intell.2014.09.013
Steinbeis, N., & Singer, T. (2014). Projecting my envy onto you: Neurocognitive mechanisms of an offline emotional egocentricity bias. NeuroImage https://doi.org/10.1016/j.neuroimage.2014.08.007
Suchy, Y. (2009). Executive functioning: Overview, assessment, and research issues for non-neuropsychologists. Annals of Behavioral Medicine https://doi.org/10.1007/s12160-009-9097-4
Syngelaki, E. M., Moore, S. C., Savage, J. C., Fairchild, G., & van Goozen, S. H. M. (2009). Executive functioning and risky decision making in young male offenders. Criminal Justice and Behavior https://doi.org/10.1177/0093854809343095
Tajfel, H., & Turner, J. (1979). An integrative theory of inter-group conflict. In The social psychology of intergroup relations.
Takahashi, H., Kato, M., Matsuura, M., Mobbs, D., Suhara, T., & Okubo, Y. (2009). When your gain is my pain and your pain is my gain: Neural correlates of envy and schadenfreude. Science https://doi.org/10.1126/science.1165604
Tangney, J. P., Stuewig, J., & Mashek, D. J. (2007). Moral emotions and moral behavior. Annual Review of Psychology https://doi.org/10.1146/annurev.psych.56.091103.070145
Timmermann, M., Jeung, H., Schmitt, R., Boll, S., Freitag, C. M., Bertsch, K., & Herpertz, S. C. (2017). Oxytocin improves facial emotion recognition in young adults with antisocial personality disorder. Psychoneuroendocrinology https://doi.org/10.1016/j.psyneuen.2017.07.483
Torralva, T., Roca, M., Gleichgerrcht, E., López, P., & Manes, F. (2009). INECO Frontal Screening (IFS): A brief, sensitive, and specific tool to assess executive functions in dementia. Journal of the International Neuropsychological Society. https://doi.org/10.1017/S1355617709990415
Ttofi, M. M., Farrington, D. P., Piquero, A. R., Lösel, F., DeLisi, M., & Murray, J. (2016). Intelligence as a protective factor against offending: A meta-analytic review of prospective longitudinal studies. Journal of Criminal Justice https://doi.org/10.1016/j.jcrimjus.2016.02.003
Vaish, A. (2018). The prosocial functions of early social emotions: the case of guilt. Current Opinion in Psychology https://doi.org/10.1016/j.copsyc.2017.08.008
van Dijk & Ouwerkerk, J. W. E. (2014). Schadenfreude: Understanding pleasure at the misfortune of others. Cambridge University Press.
Van Dijk, W. W., Ouwerkerk, J. W., Goslinga, S., Nieweg, M., & Gallucci, M. (2006). When people fall from grace: Reconsidering the role of envy in Schadenfreude. Emotion https://doi.org/10.1037/1528-3542.6.1.156
Van Wijk, A., Van Horn, J., Bullens, R., Bijleveld, C., & Doreleijers, T. (2005). Juvenile sex offenders: A group on its own? International Journal of Offender Therapy and Comparative Criminology, 49(1), 25–36.
Veneziano, C., Veneziano, L., LeGrand, S., & Richards, L. (2004). Neuropsychological executive functions of adolescent sex offenders and nonsex offenders. Perceptual and Motor Skills https://doi.org/10.2466/pms.98.2.661-674
Villemarette-Pittman, N. R., Stanford, M. S., & Greve, K. W. (2003). Language and executive function in self-reported impulsive aggression. Personality and Individual Differences https://doi.org/10.1016/S0191-8869(02)00136-8
Wade, M., Prime, H., Jenkins, J. M., Yeates, K. O., Williams, T., & Lee, K. (2018). On the relation between theory of mind and executive functioning: A developmental cognitive neuroscience perspective. Psychonomic Bulletin and Review https://doi.org/10.3758/s13423-018-1459-0
Yoder, K. J., & Decety, J. (2014). The good, the bad, and the just: Justice sensitivity predicts neural response during moral evaluation of actions performed by others. Journal of Neuroscience https://doi.org/10.1523/JNEUROSCI.4648-13.2014
Yurgelun-Todd, D. (2007). Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology https://doi.org/10.1016/j.conb.2007.03.009
Zou, Z., Meng, H., Ma, Z., Deng, W., Du, L., Wang, H., … Hu, H. (2013). Executive functioning deficits and childhood trauma in juvenile violent offenders in China. Psychiatry Research https://doi.org/10.1016/j.psychres.2012.09.013
Contributors
DFO, SB, HSG, CI, and AI conceived and designed the project, the analysis, contributed analysis tools, performed the analysis, and participated in the drafting of the final manuscript. MP and CI collected the data. DFO, SB, MPS, and AI conducted data analysis and interpretation, and participated in the drafting of the final manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
None to declare
Funding
AI is partially supported by CONICET, ANID/FONDAP (15150012), ANID/Fondecyt/Regular 1210195, PICT (2017-1818 and 2017-1820).
Author information
Authors and Affiliations
Corresponding author
Additional information
Open practice statement
None of the data for the experiments reported here is available, and none of the experiments was preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Franco-O’Byrne, D., Ibáñez, A., Santamaría-García, H. et al. Neuroanatomy of complex social emotion dysregulation in adolescent offenders. Cogn Affect Behav Neurosci 21, 1083–1100 (2021). https://doi.org/10.3758/s13415-021-00903-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00903-y