Abstract
Individuals with high intolerance of uncertainty (IU) have been shown to exhibit abnormal threat responding, which may be mediated by hyperactive anterior insula (aINS) response to uncertainty. Research has indicated that individuals with high IU also exhibit abnormal positive valence responding, suggesting that IU may impact responding to uncertainty regardless of the valence of the potential outcome. To date, no study has investigated the neural processes associated with IU and response to uncertain positive stimuli, such as rewards. Therefore, this study was designed to examine the association between individual differences in IU and neural activation during uncertain reward using functional magnetic resonance imaging (fMRI). Thirty-seven adults completed a self-report measure of IU and a reward task during fMRI. Consistent with the threat literature, greater IU was associated with increased aINS activation during uncertain reward. This association was more robust for the prospective IU subscale, a dimension characterized by worry about future events. Together with prior studies, these findings provide evidence that IU is related to abnormal threat and reward responding, and that these deficits may be similarly linked to hyperactive aINS response to uncertainty.
Similar content being viewed by others
Humans use information about past experiences and current internal and external states to make inferences about the future, which allows for the opportunity to pursue rewards and avoid dangers. If information about upcoming events is lacking, outcomes become more uncertain and the ability to prepare for future events is diminished, resulting in increased anxiety (Grupe & Nitschke, 2013). Human and nonhuman research indicates that uncertainty elicits heightened, sustained vigilance and anticipatory anxiety (Blanchard, Yudko, Rodgers, & Blanchard, 1993; Herry et al., 2007; Jackson, Nelson, & Proudfit, 2015), and that the less information there is available about an environmental situation, the more it is appraised as aversive (D’Amato, 1974; Lejuez, Eifert, Zvolensky, & Richards, 2000).
Individuals differ in their response to uncertainty. This is thought to reflect, in part, individual differences in intolerance of uncertainty (IU), defined as the tendency to respond to uncertain events and situations with negative cognitive, behavioral, and/or physiological reactions (Birrell, Meares, Wilkinson, & Freeston, 2011; Carleton, 2012; Carleton et al., 2012; Carleton, Norton, & Asmundson, 2007; Dugas, Schwartz, & Francis, 2004). Factor analytic studies of IU suggest that it consists of two distinct subfactors—inhibitory IU (I-IU) and prospective IU (P-IU). I-IU is characterized by behavioral inhibition (e.g., freezing) in response to uncertainty, whereas P-IU is characterized by concerns and worry about future events (Carleton et al., 2007; Hong & Lee, 2015; McEvoy & Mahoney, 2011). Generally, I-IU reflects behavioral disturbances whereas P-IU reflects abnormal cognitive perceptions in response to uncertainty.
It is necessary to highlight that IU was initially proposed as a core construct of generalized anxiety disorder (GAD; Dugas et al., 2004), but more recent evidence suggests that individuals across a wide range of internalizing disorders (e.g., major depressive disorder [MDD], panic disorder [PD], and obsessive-compulsive disorder [OCD]) exhibit high levels of IU (Barlow et al., 2014; Boswell, Thompson-Hollands, Farchione, & Barlow, 2013; Holaway, Heimberg, & Coles, 2006; Jacoby, Fabricant, Leonard, Riemann, & Abramowitz, 2013). High IU is therefore transdiagnostic and has been proposed as a phenotypic core of internalizing psychopathology (Barlow et al., 2014). Consequently, individual differences in IU are clinically important and are thought to contribute to exaggerated levels of negative affect and physiological arousal, and influence the maintenance of chronic anxiety and avoidance behaviors (Barlow, 2002; Greco & Roger, 2003). A better understanding of the mechanims underlying individual differences in IU may therefore lead to improved targeted, transdiagnostic interventions for internalizing psychopathology.
To date, numerous studies have examined how individual differences in IU relate to responsivity to uncertain threat. For example, studies have shown that greater levels of IU are associated with increased threat perception (Bredemeier & Berenbaum, 2008; de Bruin, Rassin, & Muris, 2006), heightened startle responding (Gorka, Lieberman, Nelson, Sarapas, & Shankman, 2014; Nelson, Liu, Sarapas, & Shankman, 2016), and exaggerated error-related brain activity (Jackson, Nelson, & Hajcak, 2016) during conditions of ambiguous or unpredictable threat. However, as was noted previously, the construct of IU is broad, and its conceptualization is not tied to the valance of the uncertain information. In other words, individual differences in IU likely relate to responding to uncertain aversive and appetitive stimuli.
In support of this idea, growing evidence indicates that IU is associated with the processing of uncertain pleasurable stimuli, such as rewards. For example, high IU is associated with poorer decision making during a delayed probabilistic reward task (Luhmann et al., 2011), decreased frontal electroencephalogram (EEG) asymmetry (a psychophysiological indicator of reduced approach motivation) during reward anticipation (Nelson, Shankman, & Proudfit, 2014), and an altered reward positivity (RewP)—an event-related potential (ERP) response to monetary gains relative to losses (Nelson, Kessel, Jackson, & Hajcak, 2015). Although dysfunctional threat and reward processing has been purported to be specific to anxiety and depressive disorders, respectively (Shankman et al., 2013), this literature suggests that both classes of disorders are associated with high IU and dysfunctional responding to uncertain threat and reward. Thus, individual differences in IU, and the impact this response tendency has on threat and reward processing, may contribute to the high comorbidity between these disorders.
Human and nonhuman neuroscience research suggests that a specific frontolimbic neural circuit mediates responding to uncertainty, which includes regions such as the amygdala (AMYG); anterior insula (aINS); bed nucleus of the stria terminalis (BNST); orbitofrontal cortex (OFC); anterior cingulate cortex (ACC); and the dorsolateral, ventrolateral, and ventromedial prefrontal cortices (dlPFC, vlPFC, vmPFC; see Grupe & Nitschke, 2013, for a review). Within this circuit, the aINS is thought to be critically involved in exaggerated physiological and subjective responses to uncertainty. Evidence indicates that the aINS integrates internal and external information to produce interoceptive awareness and facilitates the generation of anticipatory emotional responses for future-oriented events (Craig, 2009; Paulus & Stein, 2006). Related to this, the aINS uses information about interoceptive states to perceive the passage of time—a function that is central to anticipatory processes (Craig, 2011). Fundamentally, during times of uncertainty, the aINS creates a subjective response to the question, “How is this going to feel?”
As expected, it has been demonstrated that IU is positively correlated with aINS activation during uncertain threat (Shankman et al., 2014; Somerville et al., 2013), suggesting that individual differences in IU relate to individual differences in aINS response to uncertainty, and that hyperactive aINS functioning may underlie the characteristic maladaptive response styles of individuals with high IU. It is further possible that hyperactive aINS functioning contributes to the association between high IU and internalizing psychopathology as hyperactive aINS response to uncertain threat has been observed in individuals with high trait anxiety (Simmons, Strigo, Matthews, Paulus, & Stein, 2006; Stein, Simmons, Feinstein, & Paulus, 2007) and patients with anxiety disorders (Gorka, Nelson, Phan, & Shankman, 2014; Straube, Mentzel, & Miltner, 2007). Taken together, exaggerated aINS reactivity may mediate the association between individual differences in IU and anxiety symptoms, and broader internalizing psychopathology.
Studies have yet to investigate the association between IU and aINS response to uncertain reward, despite the fact that emerging evidence indicates that individuals high in IU may exhibit difficulties with uncertain negative and positive outcomes, and that high IU relates to dysfunctional reward anticipation (Nelson, Shankman, & Proudfit, 2014). Although the aINS has not traditionally been considered a key node of the neural reward circuit (Haber & Knutson, 2010), studies have reported aINS activation during reward tasks (Ernst et al., 2004; Gorka, Huggins, Fitzgerald, Nelson, Phan, & Shankman 2014), which is consistent with its broad role in interoceptive awareness and subjective future-oriented states (Craig, 2009, 2011). It has also been demonstrated that IU is positively correlated with bilateral anterior and middle insula activation during the viewing of ambiguously angry and happy faces (i.e., two appetitive emotions; Simmons, Matthews, Paulus, & Stein, 2008). As such, preliminary support suggests that IU may be related to aINS hyperactivity, although studies are critically needed to test this hypothesis to help clarify the construct of IU and elucidate the potential associations between IU and abnormal threat and reward responding.
This study was designed to examine the association between individual differences in self-reported IU and neural activation during uncertain reward using functional magnetic resonance imaging (fMRI). The sample included adult participants with a distributed range of internalizing symptoms and, relatedly, a distributed range of self-reported IU. We hypothesized that IU would be associated with increased aINS activation during uncertain reward, consistent with studies investigating the relation between IU and neural response to uncertain threat (Shankman et al., 2014; Sommerville et al., 2013). Because the construct of IU can be parsed into I-IU and P-IU, our study examined the impact of each factor separately, as well as the combined IU total score, on neural response during uncertain reward. Given the very small literature on IU subfactors, we did not have specific hypotheses about differences between I-IU and P-IU.
Method
Participants
The sample included 40 adults who were recruited from the community and enrolled in a larger study on affective and motivational processes associated with anxiety and depression (Shankman et al., 2013). Three participants did not complete the IU measure, and thus the final sample included 37 individuals with a range of internalizing symptoms and diagnoses (see Table 1). As per the aims of the larger study, individuals were excluded if they had a lifetime diagnosis of a psychotic disorder, bipolar disorder, or dementia; were unable to read or write in English; had a history of head trauma with a loss of consciousness; or were left-handed. Informed consent was obtained before participation, and the research protocol was approved by the University of Illinois–Chicago Institutional Review Board.
Self-report measures
Intolerance of Uncertainty Scale (IUS)
The IUS (Freeston et al., 1994) is a 27-item self-report measure that assesses the degree to which individuals find uncertainty to be distressing, frustrating, and undesirable. Items are rated on a 5-point Likert scale ranging from 1 (not at all characteristic of me) to 5 (entirely characteristic of me). Our study used the 12-item version of the IUS (Carleton et al., 2007), which consists of items that are not specific to any psychiatric disorder and has better psychometric properties than the full 27-item scale. The 12-item IUS (M = 28.72, SD = 11.68, skew = 0.84) produces two factor-analytically derived subscales: a 7-item Prospective IU scale (P-IU; M = 18.81, SD = 7.32, skew = 0.50) measuring fear and anxiety in response to future events, and a 5-item Inhibitory IU scale (I-IU; M = 9.91, SD = 5.35, skew = 1.0) measuring the propensity for uncertainty to inhibit one’s actions or experiences. Of note, mean IU scores (and SDs) from the current study are comparable to published reports from other heterogeneous, unselected samples (e.g., Jackson et al., 2016), and, as expected, are lower than reports from purely clinical patient samples (e.g., Jacoby et al., 2013). Both P-IU and I-IU scores were normally distributed across participants (see Fig. 1b).
Inventory of Depression and Anxiety Symptoms (IDAS)
In addition to the IUS, participants also completed the IDAS (Watson et al., 2007), a self-report measure designed to assess symptoms of internalizing psychopathology during the previous 2 weeks. Participants are asked to respond to each item using a 5-point Likert scale ranging from 1 (not at all) to 5 (extremely), and scores are summed to create subscales that are linked to DSM-IV (APA, 2000) mood and anxiety symptom profiles. The scale yields 11 specific, factor-analytically derived symptom scales: Depression, Dysphoria, Lassitude, Insomnia, Suicidality, Appetite Gain, Appetite Loss, Ill-Temper, Well-Being, Panic, Social Anxiety, and Traumatic Intrusions. Prior research has demonstrated that the IDAS has excellent psychometric properties, including internal consistently, test–retest reliability, and convergent and discriminant validity (Watson et al., 2007). Reliability of the IDAS subscales in our study was excellent (α range 0.76–0.91).
Procedure and reward task
After providing written informed consent, all participants completed a mock scan and a practice version of the experimental task. Approximately 7 days later, participants returned for the fMRI scan. All scanning sessions took place between 7 a.m. and 12 p.m., and participants were instructed to abstain from caffeine and tobacco for at least 2 hours before the scan.
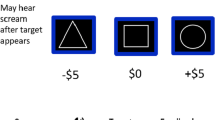
Before the task, participants were told that they would be playing a slot machine game, similar to one they would see at a casino, and had the chance to win up to $20 in cash. The task itself was a computerized, passive slot machine paradigm with two conditions—reward (R) and no incentive (NI) (see Fig. 1). In both conditions, participants were instructed to press a button, located on an fMRI compatible button box, to start the trial. A ring of geometric shapes would then “spin” on the screen and “land” on a result. Pressing the button only started the trial and in no way impacted the outcome, as the task was fixed across participants and analogous to a real-world slot machine. In the R condition, participants won money if the reel landed on an image of a birthday cake. If the reel landed on something other than the birthday cake, participants’ earnings did not change (i.e., they did not win or lose any money).
Unbeknownst to the participants, 50 % of the R trials resulted in a monetary reward ranging from $0.50 to $2.50. Participants were told before the game that the reward probabilities were random. In the NI condition, the reel would also spin and land on a result; however, the participants’ earnings did not change, no matter the outcome, and thus there was no reward incentive. In other words, participants continued to play the slot machine game, but there was no possibility of winning (or losing) money in the NI condition.
The task included 64 trials, across two runs, equally divided into R and NI (i.e., 32 trials of each condition). The current condition was always clearly indicated by a colored cue in the middle of the computer screen. For R conditions the cue was green, and for NI conditions the cue was red. Participants were provided these instructions during the practice run. For each trial, the reel would spin for 4 to 6 s. The jitter was distributed randomly and matched across R and NI trials. The result was always displayed on the screen for 3 s. The current event of interest was the reward “anticipation” phase of each trial (i.e., the 4–6 s the reel was spinning on the screen). Trials of each condition were blocked such that four trials of the same condition (R or NI) were presented consecutively, followed by a 10-s fixation cross, similar to other fMRI tasks (Nelson, et al. 2015; Reeck & Egner, 2015). There were no interstimulus intervals between trials in a given block. The total task time was approximately 12 min. At the end of the task, all participants were given their winnings of $17 in cash.
It is worth highlighting that this task was both similar and distinct from the widely used monetary incentive delay task (MID; Knutson, Adams, Fong, & Hommer, 2001; Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008; Knutson, Westdorp, Kaiser, & Hommer, 2000). Most notably, unlike the MID, this slot-machine task does not include a punishment condition in which participants lose money. There is also no behavioral performance component, and, related to such, no decision-making aspect (i.e., this task is completely passive). The task was designed more like an actual slot machine, where participants press a button and wait several seconds to see if they won money and, if so, how much they earned. The anticipation phases of the reward trials are inherently uncertain because the outcome is unknown.
fMRI acquisition
Functional MRI was performed on a 3 T GE magnetic resonance scanner at the University of Illinois–Chicago Medical Center. Functional images were acquired using a gradient-echo echo-planar images (2-s TR, 25-ms TE, 82° flip, 64 × 64 matrix, 200-mm FOV, 3-mm slice thickness, 0-mm gap, with 40 axial slices). A high-resolution, T1-weighted anatomical scan was also acquired in the same axial orientation (25° flip, 512 × 512 matrix, 220-mm FOV; 1.5-mm slice thickness; 120 axial slices).
fMRI processing and analyses
Data from all 37 participants met criteria for high quality and scan stability with minimum motion correction (i.e., less than 1.5-mm displacement in any one direction) and excellent whole-brain coverage (including the aINS). Functional data were analyzed using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuro-Science, London, UK). Images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participants’ T1 image, resampled to 2-mm3 voxels, and smoothed with an 8-mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Given the aims of this study, at the first level we modeled the spinning of the reel (i.e., anticipation phase) and the presentation of the result (i.e., outcome) separately for each trial. The model therefore included four effects modeled with box-car regressors: (1) R condition anticipation phase, (2) R condition outcome, (3) NI condition anticipation phase, and (4) NI condition outcome. Individual motion parameters were included as covariates of no interest. Effects were estimated at each voxel and for each subject.
Individual contrast maps for R anticipation greater than NI anticipation were generated for each participant. These contrast maps were then entered into a second-level one-sample t test to examine the main effect of uncertain reward on neural activation. Activations surviving family-wise error (FWE) p < .05 at the peak voxel were considered significant. As shown in detail below, results indicated that the task significantly activated the bilateral aINS as expected. Therefore, we next extracted BOLD signal responses (i.e., parameter estimates, β weights [arbitrary units]) from 10-mm (radius) spheres surrounding peak voxel activations in the left and right aINS. Using SPSS 22 (i.e., Statistical Package for the Social Science, Version 22) software, we then conducted two hierarchical linear regression analyses, one for the left aINS and one for the right aINS. Age was first entered as a covariate. P-IU and I-IU were then entered as simultaneous independent variables, which allows for examination of each IU subscale while controlling for the other. Of note, several recent studies have suggested that the two IU subscales should be entered into statistical models simultaneously to prevent potential mutual suppressor effects in which two correlated predictor variables have the opposite effect on the criterion/outcome variable, causing the associations to be obscured when each subscale is examined separately (Jackson et al., 2016; Nelson et al., 2016; Watson, Clark, Chmielewski, & Kotov, 2013).
Given the characteristics of this sample, we explored whether aINS activation was significantly associated with current diagnoses of MDD or anxiety disorder, and if aINS was correlated with current depressive and anxiety symptoms. Because panic disorder was the most prevalent anxiety disorder within the sample, we chose to examine the association between aINS and panic symptoms, specifically. Thus, partial correlation analyses (controlling for age) were conducted with IDAS Depression subscale scores, IDAS Panic subscale scores, and extracted aINS BOLD signal values.
Results
Main effect of the reward task
Whole-brain analyses indicated that uncertain reward (R condition spins > NI condition spins) significantly activated a large contiguous cluster of mesolimbic reward regions, including bilateral nucleus accumbens, caudate, and putamen (peak MNI [4, -22, -2], k = 124,624 mm3, Z = 6.87, p < .001). As expected, uncertain reward also significantly activated the bilateral aINS (see Table 2). These whole-brain results are illustrated in Fig. 1a.
IU and insula reactivity
Results from the left and right aINS regression models are presented in Table 3. Analyses revealed that during uncertain reward, greater P-IU was associated with greater left and right aINS activation. There was no association between I-IU and aINS reactivity during uncertain reward. Notably, the unique effect of P-IU and aINS activation was observed when both IU subscales were included in the model simultaneously, and when examining each IU subscale individually (see Fig. 1c and d).
To further explore the specificity of the P-IU findings, we conducted hierarchical linear regression models in which we entered the P-IU × I-IU interaction term into the third step. Results indicated a significant two-way interaction for the left (β = -1.92, t = -2.29, p < .03) and right (β = -2.00, t = -2.52, p < .02) aINS. Follow-up simple slope analyses revealed that the effect of P-IU on left and right aINS reactivity to uncertain reward was greater at low I-IU (left: β = 2.16, t = 2.96, p < .01; right: β = 2.28, t = 3.31, p < .01) relative to high I-IU (left: β = 0.59, t = 2.75, p < .05; right: β = 0.65 t = 3.19, p < .01), suggesting that P-IU is the aspect of IU that is contributing most to individual differences in aINS reactivity (Fig. 2).
(a) Voxel-wise statistical t map on a canonical brain displaying significant activations during uncertain reward anticipation > no-incentive anticipation. The t map is displayed at family-wise error (FWE) corrected p < .05 at the peak voxel with a cluster size of 20 or more contiguous voxels. (b) Scatter plot that illustrates the distribution of P-IU and I-IU self-report scores. (c) Scatter plot depicting the significant association between P-IU and bilateral anterior insula activation during uncertain reward anticipation. (d) Scatter plot depicting the nonsignificant association between I-IU and bilateral anterior insula activation during uncertain reward anticipation. For both c and d, the anterior insula values were derived by extracting BOLD signal parameter estimates from 10 mm radius spheres surrounding the peak voxels on the left and right. BOLD = blood oxygenation level dependent; P-IU = prospective intolerance of uncertainty; I-IU = inhibitory intolerance of uncertainty
Post hoc analyses
Because some individuals in the current sample had diagnoses of depression and anxiety, post hoc we examined whether the relation between P-IU and aINS activation was moderated by psychiatric diagnosis (i.e., MDD and/or anxiety) and found no significant P-IU by MDD and/or anxiety interactions (all ps > .75). Partial correlation analyses between symptoms and aINS activation (controlling for age) did reveal that across all subjects, current depressive (r = .38, p < .03; see Fig. 3a) and panic symptoms (r = .37, p < .03; see Fig. 3b) were significantly associated with greater right aINS reactivity during uncertain reward. Partial correlations between the left aINS and symptoms were not statistically significant, but were considered a trend, and in the same direction as above (left aINS—Depression: r = .31, p = .07; left aINS—Panic: r = .29, p = .09). Notably, both depressive and panic symptoms were significantly correlated with individual differences in P-IU (Depression: r = .68, p < .01; Panic: r = .41, p < .01) and I-IU (Depression: r = .68, p < .01; Panic: r = .32, p = .06), consistent with the existing literature. In light of these positive associations, we next tested whether right aINS activation mediated the association between P-IU and depression and panic symptoms using PROCESS—an SPSS macro for path-analysis-based modeling (Hayes, 2013). P-IU was specified as the independent variable, right aINS activation as the mediator, and panic or depressive symptoms (two separate models) as the dependent variable. Age was included as a covariate consistent with above. Results indicated that neither mediation model was significant (Depression model: b = -0.02, 95 % CI [-0.66, - 0.49]; Panic model: b = 0.07, 95 % CI [-0.07, - 0.27]). Although the analyses indicated significant “a” (i.e., P-IU → aINS reactivity) and “c” paths (i.e., P-IU → depression/panic symptoms), there was no significant “b” path corresponding to no significant association between right aINS reactivity and symptoms while controlling for P-IU (ps > .30).
Discussion
Growing evidence supports IU as a transdiagnostic construct associated with maladaptive responding to uncertain threat and reward (Nelson et al., in press; Shankman et al., 2014). Data also indicate that, in the context of uncertain threat, those with high IU exhibit heightened aINS reactivity (Simmons et al., 2008), which may contribute to maladaptive cognitive and behavioral responding (Grupe & Nitschke, 2013). The aim of this study was to extend this line of work and examine whether individual differences in IU were also associated with heightened aINS reactivity during uncertain reward. In a sample of adults with a distributed range of IU, our findings indicate that greater levels of IU were indeed associated with more aINS activation during uncertain reward, and that this finding was more robust in regards to the P-IU subscale relative to the I-IU subscale. Exploratory analyses revealed that in addition to P-IU, aINS reactivity was positively associated with current depressive and panic symptoms; however, models testing whether aINS reactivity mediated the association between P-IU and depression/panic were not significant, and, thus, the P-IU and aINS association was independent of the depression/panic symptoms and aINS association. Interpretations and implications of these results are discussed below.
This study indicates that greater levels of IU are associated with more aINS activation during uncertain reward. This is consistent with prior studies noting that greater levels of IU are associated with greater aINS activation during uncertain threat (Shankman et al., 2014; Somerville et al., 2013). It is also consistent with the developing view that individuals with high IU exhibit maladaptive, abnormal responses in the context of uncertainty regardless of the valence of the potential event or outcome, which may be mediated by exaggerated aINS reactivity. In other words, those with high IU may find all (or most) forms of uncertainty distressing and/or arousing, and have a tendency to over-engage the aINS in contexts in which information about future outcomes is limited. In regard to this study, those with high levels of IU (particularly P-IU) may have found the anticipation of an uncertain rewarding outcome (i.e., “winning” money) to be distressing or over-arousing—as evidenced by exaggerated aINS activation. This subjective distress and hyperactive neural response could have diminished the hedonic and approach-eliciting aspects of reward. Indeed, a prior study by our group demonstrated that individual differences in IU mediated the association between depression and diminished approach motivation during a similar reward task (Nelson et al., 2014). Therefore, high IU appears to be an individual difference factor that negatively impacts both threat and reward responding.
Considering these findings and the broader threat/reward literature, it is possible that high IU may contribute to the affective deficits that are characteristic of both anxiety and depression via alterations in aINS functioning (Barlow et al., 2014). More specifically, in response to uncertainty, individuals with high IU may exhibit abnormal neural responding (i.e., aINS hyperactivity), which impairs appraisal and anticipatory processes and facilitates the generation and maintenance of internalizing symptoms. In this study, individual differences in IU, depression, anxiety, and aINS reactivity during uncertain reward were all moderately correlated. This indicates that these factors are indeed interrelated and that P-IU may contribute to internalizing psychopathology by impacting the neural processing of uncertain outcomes. We attempted to more formally test this hypothesis by examining whether aINS hyperactivity mediated the association between IU and current depression and/or panic symptoms. The mediation models were nonsignificant, but because the current study was cross-sectional and included a relatively small sample, these null findings should be interpreted with caution. Future studies are needed to continue to investigate the role of IU in both threat and reward processing, and further, how these processes relate to the risk and progression of disorders like depression and anxiety.
The association between IU and aINS activation was more robust in regards to the P-IU subscale. Prospective IU is conceptualized as a cognitively focused dimension that reflects the tendency to experience fear, anxiety, and worry during uncertain outcomes, whereas inhibitory IU is a behaviorally focused dimension that reflects the tendency to suppress or inhibit behavior in response to uncertain situations (Carleton et al., 2012; McEvoy & Mahoney, 2011). P-IU and I-IU are therefore considered different manifestations of the broader IU construct. P-IU is characterized by hyperreactivity (rather than inhibition) and more closely maps onto anticipatory processes and the design of the current reward task in which participants anticipated the potential receipt of (future) monetary rewards. P-IU is therefore directly related to the anticipation of uncertain outcomes, and it is expected that this dimension be related to individual differences during unpredictable rewards. Nelson et al. (2014) also found that the P-IU subscale, but not the I-IU subscale, was associated with reduced approach motivation and mediated the association between depression and approach motivation during reward. Other studies have found similar results in which P-IU was uniquely associated with diminished reward or exaggerated threat responding (Jackson et al., 2016; Nelson et al., 2016). However, two studies have had slightly different results. First, during the anticipation of unpredictable negative images, Shankman et al. (2014) found that aINS activation was more robustly correlated with I-IU than P-IU scores. Meanwhile, Somerville et al. (2013) found that IU total scores were positively correlated with aINS activation during a threat task very similar to that used by Shankman et al. (2014), but did not examine the independent effects of each IU subscale. Therefore, it is possible that P-IU may indeed be more related to increased aINS responding to uncertainty, though future studies are needed to further clarify whether different dimensions of IU differentially relate to neural response during uncertain threat and reward.
Although our findings address important gaps in the IU literature, there are several limitations. First and foremost, the two fMRI task conditions (i.e., R anticipation and NI anticipation) differ on predictability and the potential for reward. Therefore, it is possible that individual differences in IU may relate to neural reactivity to uncertain reward, specifically, and/or reward processing more broadly. Future studies that implement tasks that carefully manipulate uncertainty, and directly tap into the intolerance of uncertainty construct, will be crucial in building upon these preliminary findings. For instance, subsequent studies should consider tasks that allow for a comparison of neural reactivity during the anticipation of unpredictable rewards relative to predictable rewards (Alkozei, Smith, & Killgore, 2016), which could involve manipulations of reward timing, probability, and/or amount. Second, to better simulate dynamics of the real world, studies could also consider tasks that manipulate rewards (or threats) in such a way that participants are able to learn various contingencies, make decisions regarding the potential for reward, and then use these estimates to inform their behavioral response (e.g., Hefner, Starr, & Curtin, 2015; Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005). This would ultimately allow for real-time assessments of aversion/preference for uncertainty and its impact on behavior. On a day-to-day basis, individuals often approach situations with some knowledge about past experiences and risk and reward likelihood, and this knowledge guides decisions about pursuing rewards and/or avoiding dangers. It is posited that individuals with high and low IU differ in how they interpret ambiguity, evaluate risk and reward, and subsequently behave within the context of uncertainty, which maintains maladaptive affective response styles (Barlow et al., 2014). Thus, the more that studies seek to capture the unfolding of these various processes during both threat and reward (in the same sample), the better we will be able to identify and define the IU profile and its neural correlates.
In addition to the limitations regarding task design, it is also necessary to highlight that the current sample size was small, which likely reduced statistical power. Second, approximately half of the participants had diagnoses of MDD and/or anxiety disorders. Although this increased the variability within our self-reported IU measure, and the pattern of results was not moderated by diagnosis, it is unclear whether our findings would generalize to other samples (e.g., purely healthy controls, individuals with primarily externalizing disorders). Third, five of the current participants were taking psychiatric medications that may have impacted their neural responding; however, removing those five did not change the current results. Last, we did not collect self-report ratings during the reward task, and it is unclear if individual differences in IU were related to subjective response during the reward-anticipation task. It will be important for future studies to link these neural findings to functional (e.g., behavioral, affective, cognitive) outcomes within individuals with high IU.
This study indicated that greater levels of IU were associated with more aINS activation during uncertain reward. This is consistent with a growing literature suggesting that individuals with high IU may exhibit maladaptive cognitive and behavioral responses to uncertain outcomes, regardless of the valence of the outcome. It also suggests that these individual differences may be linked to hyperactive aINS responsivity during uncertain contexts. Because high IU has recently been proposed as a potential core, transdiagnostic construct implicated in abnormal threat and reward responding, it is important that future studies continue to investigate the psychophysiological processes that mediate these deficits and how IU contributes to the pathophysiology of depression and anxiety.
References
Alkozei, A., Smith, R. S., & Killgore, W. D. (2016). Exposure to blue wavelength light modulates anterior cingulate cortex activation in response to ‘uncertain’ versus ‘certain’ anticipation of positive stimuli. Neuroscience Letters, 616, 5–10.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, D.C: American Psychiatric Association.
Barlow, D. H. (2002). Anxiety and its disorders (2nd ed.). New York, NY: Guilford Press.
Barlow, D. H., Sauer-Zavala, S., Carl, J. R., Bullis, J. R., & Ellard, K. K. (2014). The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science, 2, 344–365.
Birrell, J., Meares, K., Wilkinson, A., & Freeston, M. (2011). Toward a definition of intolerance of uncertainty: A review of factor analytical studies of the Intolerance of Uncertainty Scale. Clinical Psychological Review, 31, 1198–1208.
Blanchard, R. J., Yudko, E. B., Rodgers, R. J., & Blanchard, D. C. (1993). Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behavioral and Brain Research, 58, 155–165.
Boswell, J. F., Thompson-Hollands, J., Farchione, T. J., & Barlow, D. H. (2013). Intolerance of uncertainty: A common factor in the treatment of emotional disorders. Journal of Clinical Psychology, 69, 630–645.
Bredemeier, K., & Berenbaum, H. (2008). Intolerance of uncertainty and perceived threat. Behaviour Research and Therapy, 46(1), 28–38.
Carleton, R. N. (2012). The intolerance of uncertainty construct in the context of anxiety disorders: Theoretical and practical perspectives. Expert Review of Neurotherapeutics, 12(8), 937–947.
Carleton, R. N., Norton, M. A. P. J., & Asmundson, G. J. G. (2007). Fearing the unknown: A short version of the Intolerance of Uncertainty Scale. Journal of Anxiety Disorders, 21, 105–117.
Carleton, R. N., Weeks, J. W., Howell, A. N., Asmundson, G. J., Antony, M. M., & McCabe, R. E. (2012). Assessing the latent structure of the intolerance of uncertainty construct: An initial taxometric analysis. Journal of Anxiety Disorders, 26(1), 150–157.
Craig, A. D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70.
Craig, A. D. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82.
D’Amato, M. R. (1974). Derived motives. Annual Review of Psychology, 25, 83–106.
de Bruin, G. O., Rassin, E., & Muris, P. (2006). Worrying in the lab: Does intolerance of uncertainty have predictive value? Behaviour Change, 23(02), 138–147.
Dugas, M. J., Schwartz, A., & Francis, K. (2004). Brief report: Intolerance of uncertainty, worry, and depression. Cognitive Therapy and Research, 28(6), 835–842.
Ernst, M., Nelson, E. E., McClure, E. B., Monk, C. S., Munson, S., Eshel, N., … Pine, D. S. (2004). Choice selection and reward anticipation: An fMRI study. Neuropsychologia, 42(12), 1585–1597.
Freeston, M. H., Rhéaume, J., Letarte, H., Dugas, M. J., & Ladouceur, R. (1994). Why do people worry? Personality and Individual Differences, 17, 791–802.
Gorka, S. M., Huggins, A. A., Fitzgerald, D. A., Nelson, B. D., Phan, K. L., & Shankman, S. A. (2014). Neural response to reward anticipation in those with depression with and without panic disorder. Journal of Affective Disorders, 164, 50–56.
Gorka, S. M., Lieberman, L., Nelson, B. D., Sarapas, C., & Shankman, S. A. (2014). Aversive responding to safety signals in panic disorder: The moderating role of intolerance of uncertainty. Journal of Anxiety Disorders, 28(7), 731–736.
Gorka, S. M., Nelson, B. D., Phan, K. L., & Shankman, S. A. (2014). Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biology of Mood & Anxiety Disorders, 4(1), 9.
Greco, V., & Roger, D. (2003). Uncertainty, stress, and health. Personality and Individual Differences, 34(6), 10571068.
Grupe, D. W., & Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501.
Haber, S. N., & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26.
Hayes, A.F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press.
Hefner, K. R., Starr, M. J., & Curtin, J. J. (2015). Altered subjective reward valuation among drug-deprived heavy marijuana users: Aversion to uncertainty. Journal of Abnormal Psychology, 125, 138–150.
Herry, C., Bach, D. R., Esposito, F., Di Salle, F., Perrig, W. J., Scheffler, K., … Seifritz, E. (2007). Processing of temporal unpredictability in human and animal amygdala. The Journal of Neuroscience, 27, 5958–5966.
Holaway, R. M., Heimberg, R. G., & Coles, M. E. (2006). A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. Journal of Anxiety Disorders, 20, 157–174.
Hong, R. Y., & Lee, S. S. (2015). Further clarifying prospective and inhibitory intolerance of uncertainty: Factorial and construct validity of test scores from the Intolerance of Uncertainty Scale. Psychological Assessment, 27(2), 605–620.
Hsu, M., Bhatt, M., Adolphs, R., Tranel, D., & Camerer, C. F. (2005). Neural systems responding to degrees of uncertainty in human decision-making. Science, 310(5754), 1680–1683.
Jackson, F., Nelson, B. D., & Hajcak, G. (2016). The uncertainty of errors: Intolerance of uncertainty is associated with error-related brain activity. Biological Psychology, 113, 52–58.
Jackson, F., Nelson, B. D., & Proudfit, G. H. (2015). In an uncertain world, errors are more aversive: Evidence from the error-related negativity. Emotion, 15(1), 12–16.
Jacoby, R. J., Fabricant, L. E., Leonard, R. C., Riemann, B. C., & Abramowitz, J. S. (2013). Just to be certain: Confirming the factor structure of the Intolerance of Uncertainty Scale in patients with obsessive-compulsive disorder. Journal of Anxiety Disorders, 27(5), 535–542.
Knutson, B., Adams, C. M., Fong, G. W., & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience, 21(16), RC159. 1–5.
Knutson, B., Bhanji, J. P., Cooney, R. E., Atlas, L. Y., & Gotlib, I. H. (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–692.
Knutson, B., Westdorp, A., Kaiser, E., & Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12, 20–27.
Lejuez, C. W., Eifert, G. H., Zvolensky, M. J., & Richards, J. B. (2000). Preference between onset predictable and unpredictable administrations of 20% carbon-dioxide-enriched air: Implications for better understanding the etiology and treatment of panic disorder. Journal of Experimental Psychology: Applied, 6, 349–358.
Luhmann, C. C., Ishida, K., & Hajcak, G. (2011). Intolerance of uncertainty and decisions about delayed, probabilistic rewards. Behavior Therapy, 42(3), 378–386.
McEvoy, P. M., & Mahoney, A. E. (2011). Achieving certainty about the structure of intolerance of uncertainty in a treatment-seeking sample with anxiety and depression. Journal of Anxiety Disorders, 25(1), 112–122.
Nelson, B. D., Bjorkquist, O. A., Olsen, E. K., & Herbener, E. S. (2015). Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: An fMRI investigation. Psychiatry Research: Neuroimaging, 234(3), 285–291.
Nelson, B. D., Kessel, E. M., Jackson, F., & Hajcak, G. (2015). The impact of an unpredictable context and intolerance of uncertainty on the electrocortical response to monetary gains and losses. Cognitive, Affective, & Behavioral Neuroscience, 16(1), 1–11.
Nelson, B. D., Liu, H., Sarapas, C., & Shankman, S. A. (2016). Intolerance of uncertainty mediates the relationship between panic and the startle reflex in anticipation of unpredictable threat. Journal of Experimental Psychopathology. doi:10.5127/jep.048115
Nelson, B. D., Shankman, S. A., & Proudfit, G. H. (2014). Intolerance of uncertainty mediates reduced reward anticipation in major depressive disorder. Journal of Affective Disorders, 158, 108–113.
Paulus, M. P., & Stein, M. B. (2006). An insular view of anxiety. Biological Psychiatry, 60(4), 383–387.
Reeck, C., & Egner, T. (2015). Emotional task management: Neural correlates of switching between affective and non-affective task-sets. Social Cognitive and Affective Neuroscience, 10(8), 1045–1053.
Shankman, S. A., Gorka, S. M., Nelson, B. D., Fitzgerald, D. A., Phan, K. L., & O’Daly, O. (2014). Anterior insula responds to temporally unpredictable aversiveness: An fMRI study. Neuroreport, 25(8), 596–600.
Shankman, S. A., Nelson, B. D., Sarapas, C., Robison-Andrew, E. J., Campbell, M. L., Altman, S. E., … Gorka, S. M. (2013). A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology, 122(2), 322.
Simmons, A., Matthews, S. C., Paulus, M. P., & Stein, M. B. (2008). Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neuroscience Letters, 430(2), 92–97.
Simmons, A., Strigo, I., Matthews, S. C., Paulus, M. P., & Stein, M. B. (2006). Anticipation of versive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry, 60(4), 402–409.
Somerville, L. H., Wagner, D. D., Wig, G. S., Moran, J. M., Whalen, P. J., & Kelley, W. M. (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex, 23(1), 49–60.
Stein, M. B., Simmons, A. N., Feinstein, J. S., & Paulus, M. P. (2007). Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry, 164(2), 318–327.
Straube, T., Mentzel, H. J., & Miltner, W. H. (2007). Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. NeuroImage, 37(4), 1427–1436.
Watson, D., Clark, L. A., Chmielewski, M., & Kotov, R. (2013). The value of suppressor effects in explicating the construct validity of symptom measures. Psychological Assessment, 25, 929–941.
Watson, D., O’Hara, M. W., Simms, L. J., Kotov, R., Chmielewski, M., McDade-Montez, E. A., … & Stuart, S. (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19(3), 253–268.
Acknowledgments
This work was supported by grants from the Brain and Behavior Research Foundation and National Institute of Mental Health (R21 MH080689; PI: Shankman).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gorka, S.M., Nelson, B.D., Phan, K.L. et al. Intolerance of uncertainty and insula activation during uncertain reward. Cogn Affect Behav Neurosci 16, 929–939 (2016). https://doi.org/10.3758/s13415-016-0443-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-016-0443-2