Abstract
Emotional stimuli receive prioritized attentional and motoric processing in the brain. Recent data have indicated that emotional stimuli enhance activity in the cervical spinal cord as well. In the present study, we used fMRI to investigate the specificity of this emotion-dependent spinal cord activity. We examined whether the limb depicted in a passively viewed image (upper vs. lower) differentially influenced activity in the cervical segments that innervate the upper limbs, and whether this effect was enhanced by emotion. Participants completed four fMRI runs: neutral–upper limb, neutral–lower limb, negative–upper limb, and negative–lower limb. The results indicated main effects of limb and emotion, with upper limbs and negative stimuli eliciting greater activity than lower limbs and neutral stimuli, respectively. For upper-limb runs, negative stimuli evoked more activity than did neutral stimuli. Additionally, negative stimuli depicting upper limbs produced stronger responses than did negative stimuli depicting lower limbs. These results suggest that emotional stimuli augment limb-specific responses in the spinal cord.
Similar content being viewed by others
Emotions facilitate the execution of motoric responses (Koganemaru, Domen, Fukuyama, & Mima, 2012), allowing an individual to approach or avoid salient stimuli (e.g., Davidson & Irwin, 1999). Recent neuroimaging data have allowed researchers to delineate many of the neural substrates underlying this emotional modulation of movement. Multiple studies have detected increased responsivity in the supplementary motor area during the perception of emotionally arousing stimuli (e.g., Oliveri et al., 2003; Schienle, Schäfer, Walter, Stark, & Vaitl, 2005), particularly when an ongoing movement is interrupted by an emotional stimulus (Sagaspe, Schwartz, & Vuilleumier, 2011). Pereira et al. (2010) also noted that the midcingulate cortex is involved in the integration of the perception of negatively valenced emotional information and the performance of movements. These results have been complemented by functional connectivity analyses indicating that fear-relevant stimuli increase the connectivity between the amygdala and both the inferior occipital gyrus and the premotor cortex, suggesting a functional link between the structures related to fear, perception, and movement (Tettamanti et al., 2012). Additionally, studies utilizing transcranial magnetic stimulation (TMS) have consistently noted increased sensitivity in the motor cortex during emotional perception, suggesting an increase in the excitability of the corticospinal tract (Coelho, Lipp, Marinovic, Wallis, & Riek, 2010; Coombes at al., 2009; Hajcak et al., 2007; Schutter, Hofman, & Van Honk, 2008; van Loon, van den Wildenberg, van Stegeren, Hajcak, & Ridderinkhof, 2010). Together, these studies provide insights into the brain regions involved in emotion–movement interactions. However, to date, little research has examined the role of the spinal cord in these emotion-dependent responses, despite the fact that this structure is the neuroanatomical link between the nervous system regions involved in the planning and execution of these movements.
The emotional modulation of spinal cord activity was recently investigated in a study utilizing functional magnetic resonance imaging (fMRI) of the spinal cord. Smith and Kornelsen (2011) imaged the cervical spinal cord during the presentation of emotionally negative (threatening and/or gory), positive (erotic), or neutral (landscape or cityscape) photographs. For half of the trials, the participants were instructed to make buttonpress responses to the images, indicating whether the depicted scene was indoors or outdoors. Other trials required only passive viewing of the images (i.e., no motoric response). The results showed that negative stimuli evoked greater activity in cervical spinal cord segments C5–C8 than did positive or neutral stimuli. This effect of negative emotions was particularly robust when coupled with an active-motor-response task, despite the fact that the identical motoric action was performed in response to all stimulus types. This result makes evolutionary sense, as many negative emotional stimuli would elicit a protective response using the hands; consistent with this view, the largest effects found in this study were in lower cervical spinal cord segments, areas related to the control of the hands. However, the stimuli used in this initial study did not control for the type of movement implied in the images; therefore, conclusions about the motoric plans implied by the increased neural activity were speculative. By utilizing a stimulus set that better controlled for the required response (i.e., upper- vs. lower-limb movement), it would be possible to determine the specificity of the emotional modulation of spinal cord activity.
In the present study, we used spinal fMRI to compare neural responses in the cervical spinal cord during passive viewing of images depicting either upper (hands and arms) or lower (feet and legs) limbs in emotionally negative or emotionally neutral scenarios. To this end, four different image sets were created: hand–negative, hand–neutral, foot–negative, and foot–neutral. Given that neuronal innervation of the upper limbs is routed through synapses within segments of the cervical spinal cord, whereas innervation of the lower limbs is routed through synapses within segments of the lumbar spinal cord, we would expect that images depicting the upper limbs—particularly in response to emotional stimuli—would evoke greater activity in the cervical spinal cord than would images depicting the lower limbs.
An assumption underlying this experimental design is that each of the images implies that a movement should be made. This assumption was confirmed with pilot testing. However, it is also possible that a participant might produce a “freezing response” to one or more of the images. Although such a response is qualitatively different from a retraction movement or a defensive response, the limb specificity of the stimuli in the present study would still suggest that a larger response would occur for the limb involved in the photograph. Importantly, such a response would still produce detectable neuronal activity, as a freezing response involves co-contraction of agonist and antagonist muscle groups, and thus activity in the motoric nuclei of the spinal cord.
Within the cross-section of the spinal cord, descending motoric input from the brain connects with several different regions of the cord. Most motoric processing occurs in neurons in the ventral region of each spinal cord segment. However, motoric information is also relayed by synapses with interneurons in the dorsomedial region. Some of these interneurons connect directly to motor neurons, whereas others project to additional interneurons in the ventral horn (Kiernan, 1998). On the basis of this distributed representation of motoric programming, in the present study we predicted robust ventral cord activity and more diffuse dorsomedial activations in response to visual depictions of movement. As previous research has shown that emotional stimuli enhance spinal cord responses (Smith & Kornelsen, 2011), we expected this pattern to be more pronounced for negative than for neutral stimuli, particularly in the lower cervical segments related to movement of the hands (C6/C7). Given the relative paucity of data examining emotional influences on the spinal cord, it may be too soon to predict more specific patterns of emotion-dependent activity in the cross-section of the spinal cord. Instead, the aim of the present exploratory study was to compare activity levels across experimental conditions.
On the basis of these patterns of neuronal innervation, we tested four hypotheses related to the neural responses of spinal cord segments C5–C8: two main effects and two condition contrasts. We expected to see, first, a main effect of limb specificity, resulting in more neuronal activity during viewing of upper than of lower limbs. Detecting such a difference would confirm that our paradigm is logically sound—simply viewing an image implying movement can produce different activity in upper- and lower-limb conditions. We also expected to find a main effect of emotional valence, such that emotionally negative stimuli would elicit more activity than emotionally neutral stimuli. Detecting this difference would allow us to replicate the results of Smith and Kornelsen (2011), but with our new stimulus set. It would therefore demonstrate that our participants responded differently to emotional and neutral images, and that this difference was detectable using spinal fMRI. Once the paradigm and the emotionality of the stimuli were verified, it would be possible to examine limb-specific effects of emotion, the critical hypotheses of the present study. For our specific condition contrasts, we expected to observe both more activity for hand–negative than for hand–neutral images and more activity for hand–negative than for foot–negative images. Such results would indicate that the spinal cord receives input related to limb-specific motoric responses to emotional stimuli.
Method
Participants
Fourteen healthy undergraduate students (seven female, seven male; age range 18–24 years) were recruited from the University of Winnipeg. All of the participants provided informed written consent as per the ethical requirements of the National Research Council Ethics Board and the University of Winnipeg Senate Committee on Ethics in Human Research and Scholarship (SCEHRS). Participants underwent MRI safety screening, and all received a $25 honorarium for their participation.
Stimuli
The stimuli for the experiment consisted of 180 images of either negative or neutral emotional valence. Half of the images (90) depicted scenes suggestive of upper-limb (hand and arm) movement, while the other half were suggestive of lower-limb (foot and leg) movement. Within the categories of either upper- or lower-limb-specific movement, half of the images (45) were of negative valence and half were of neutral valence. The images were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 1997), supplemented by images from other publicly available sources. Due to the small number of negatively valenced hand and foot photographs available, a small number of the images were staged photos. Images were standardized such that the viewing angle and implied movement (e.g., limb retraction) were similar for pairs of negative and neutral images. For instance, if a negative image depicted a foot about to step on a nail, a neutral image depicted a foot about to step on a piece of candy.
All images were pilot tested with a separate group of undergraduate students (n = 10) in order to ensure that they elicited the appropriate emotion (negative or neutral). With ratings of 1–10 (with 10 representing the most extreme negative rating), we observed means of 4.54 for negative lower-limb images, 5.03 for negative upper-limb images, 1.43 for neutral lower-limb images, and 1.35 for neutral upper-limb images. These initial ratings for the images were used to guide the omission and replacement of emotionally ineffectual stimuli with more appropriate images, selected on the basis of their more specific relation to the target valence. Of the initially ranked stimuli, ten negative (three upper-limb) and nine neutral (five upper-limb) stimuli were replaced with more emotionally distinctive images. Each participant in the pilot study was also asked to identify any image whose implied motoric response was ambiguous; this ensured that all images in the upper-limb condition implied upper-limb movements and all images in the lower-limb condition implied lower-limb movements. All of the images were 320 × 240 pixels in size. Images were projected onto a screen behind the scanner and viewed through a mirror attached to the head coil, positioned so as to allow the participant to see the images.
Experimental design
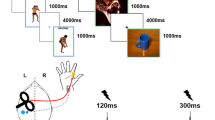
The experiment consisted of four runs, each lasting 319 s in duration. The participants were instructed to view the images on the screen, without being required to make any motoric responses. Indeed, all participants were instructed to refrain from making any movements and were visually monitored to verify that they complied with these instructions. Separate fMRI runs were conducted for each valence type (negative or neutral) and targeted limb (hand or foot), which resulted in four different runs: hand–negative, hand–neutral, foot–negative, and foot–neutral. The runs were presented in a pseudocounterbalanced order across subjects, and the images within a given run were randomized. Within each run, images were presented in a box car paradigm. Stimulus blocks 53 s in duration were composed of 15 individual photographs shown for 3,533 ms each; these blocks were alternated with 40-s rest blocks (i.e., a black fixation cross on a white background).
fMRI scanning parameters
Data were acquired in a 3 T Siemens scanner located in the Institute for Biodiagnostics, a branch of the National Research Council Canada. The images were acquired using a single-shot fast spin-echo scanning sequence with partial Fourier sampling (HASTE) with the following parameters: TE = 39 ms, TR = 1,012 ms per slice, resolution = 1.04 × 1.04 mm, FOV 200 × 100 mm, 50 % phase encode FOV. Nine 2-mm-thick contiguous sagittal slices were acquired per volume, centered on the C5 vertebra and spanning the cervical cord segments. A total of 35 volumes were acquired for each run.
Data analysis
The data were analyzed using custom-written MATLAB scripts (Stroman, 2005). The initial step of preprocessing the raw data began with the identification of the anterior, posterior, left, and right edges of the cord. Spatial normalization was accomplished by drawing a line to mark the anterior edge of the cord from the bottom of the pons to the C7/T1 disc. Next, lines on the posterior, left, and right edges were marked and manually adjusted using both the sagittal and axial views of the cord. Preprocessing was performed, including 3-D motion correction with a correlation threshold of .85, spatial smoothing at a width of 3 mm in the rostro-caudal direction, and removal of cerebral spinal fluid flow artifacts (Stroman, Bosma, & Tsyben, 2012). Next, each data set was run through an individual-level general linear model (GLM) for the paradigm described above (blocks of rest and stimulus presentation). The analyzed individual data sets were then included in a group-level random-effects analysis to quantitatively compare differences in the patterns of activity between conditions. Contrasts were performed to evaluate the main effects of emotional valence and limb specificity. Analyses were also performed to contrast between specific conditions. As noted above, planned contrasts were performed for (1) hand–negative > hand–neutral and (2) hand–negative > foot–negative. An additional contrast, (3) foot–negative > foot–neutral, was performed to determine whether emotion also had a generalized, non-limb-specific effect on spinal cord activity. Contrasts were represented as t-score maps with a t ≥ 3.0 (p ≤ .0029, df = 27 for main effects [Figs. 2 and 3 below], and p ≤ .0051, df = 13, for the single-condition planned comparisons [Figs. 4 and 5]). These contrast maps indicate regions where neuronal activity was significantly greater for one condition or effect than the other. The resulting maps displayed activated voxels such that each condition’s influence appeared in a different color. Thus, each active voxel represents a statistically significant difference in signal intensity between the two conditions, and not absolute activity. This differentiation in color for contrasting conditions allowed for visual inspection of the distinct influences of either condition and for the quantification of the comparison. For the sake of clarity, only the activated regions for the condition of greater effect are displayed in Figs. 2–5.
Time course graph of percentages of signal change for all conditions. The time course of the signal intensity (with standard error bars) for all active voxels identified with the random-effects analysis for all participants across time (in seconds) is shown. The correspondences of the changes in signal intensity are observable for each of the four conditions (see the legend for color-coded identifications) and for the stimulation paradigm (dark line)
Viewing images of upper limbs elicits greater levels of activity than does viewing images of lower limbs. (A) Example stimuli representing the contrast for the main effect of limb. In the contrast, we examined areas of the spinal cord where activity was significantly greater during viewing of upper-limb images (see the negative- and neutral-valence images depicted on the left) as compared to lower-limb images (see the negative- and neutral-valence images depicted on the right). (B) Contrast map in the axial view, showing a cross-section of C5–C8, the segments with the highest concentrations of activity across conditions. Areas of greater activity during viewing of upper- than of lower-limb images appear in yellow in the online version. Axial images are oriented according to radiological convention, with dorsal toward the bottom of each frame, the left side of the cord to the right of each frame, and from rostral to caudal from the left to the right of each row. (C) Contrast map in the sagittal view of the spinal cord, with areas of greater activity during viewing of upper- than of lower-limb images appearing in yellow in the online version. Slices progress from left to right in the map, showing the right side of the cord farthest to the left in the image, rostral toward the top, and the dorsal aspect to the right of each slice. Each slice spans C5–C8. All activity met a significance level of p ≤ .0029
Viewing images of negative valence elicits greater levels of activity than does viewing images of neutral valence. (A) Example stimuli representing the contrast for the main effect of emotional valence. In the contrast, we examined areas of the spinal cord where activity was significantly greater during viewing of negative-valence images (see the upper- and lower-limb images depicted on the left) as compared to neutral-valence images (see the upper- and lower-limb images depicted on the right). (B) Contrast map in the axial view, showing a cross-section of C5–C8, the segments with the highest concentrations of activity across conditions. Areas of greater activity during viewing of negative than of neutral images appear in yellow in the online version. Axial images are oriented according to radiological convention, with dorsal toward the bottom of each frame, the left side of the cord to the right of each frame, and from rostral to caudal from the left to the right of each row. (C) Contrast map in the sagittal view of the spinal cord, with areas of greater activity during viewing of negative than of neutral images appearing in yellow in the online version. Slices progress from left to right in the map, showing the right side of the cord farthest to the left in the image, rostral toward the top, and the dorsal aspect to the right of each slice. Each slice spans C5–C8. All activity met a significance level of p ≤ .0029
Viewing negative upper-limb images elicits greater levels of activity than does viewing neutral upper-limb images. (A) Example stimuli representing the contrast for the planned comparison of negative upper-limb images greater than neutral upper-limb images. In the contrast, we examined areas of the spinal cord where activity was significantly greater during viewing of negative upper-limb images (example image depicted on the left) as compared to neutral upper-limb images (example image depicted on the right). (B) Contrast map in the axial view, showing a cross-section of C5–C8, the segments with the highest concentrations of activity across conditions. Areas of greater activity during the viewing of negative than of neutral upper-limb images appear in yellow in the online version. Axial images are oriented according to radiological convention, with dorsal toward the bottom of each frame, the left side of the cord to the right of each frame, and from rostral to caudal from the left to the right of each row. (C) Contrast map in the sagittal view of the spinal cord, with areas of greater activity during viewing of negative than of neutral upper-limb images appearing in yellow in the online version. Slices progress from left to right in the map, showing the right side of the cord farthest to the left in the image, rostral toward the top, and the dorsal aspect to the right of each slice. Each slice spans C5–C8. All activity met a significance level of p ≤ .0051
Viewing negative upper-limb images elicits greater levels of activity than does viewing negative lower-limb images. (A) Example stimuli representing the contrast for the planned comparison of negative upper-limb images greater than negative lower-limb images. In the contrast, we examined areas of the spinal cord where activity was significantly greater during viewing of negative upper-limb images (example image depicted on the left) as compared to negative lower-limb images (example image depicted on the right). (B) Contrast map in the axial view, showing a cross-section of C5–C8, the segments with the highest concentrations of activity across conditions. Areas of greater activity during the viewing of negative upper-limb images than of negative lower-limb images appear in yellow in the online version. Axial images are oriented according to radiological convention, with dorsal toward the bottom of each frame, the left side of the cord to the right of each frame, and from rostral to caudal from the left to the right of each row. (C) Contrast map in the sagittal view of the spinal cord, with areas of greater activity during viewing of negative upper-limb images than of negative lower-limb images appearing in yellow in the online version. Slices progress from left to right in the map, showing the right side of the cord farthest to the left in the image, rostral toward the top, and the dorsal aspect to the right of each slice. Each slice spans C5–C8. All activity met a significance level of p ≤ .0051
In addition, the signal intensity of the active voxels was used to produce a graph of the intensity changes across time (in seconds) during the study, to aid in visualizing the correspondence of the changes in signal intensity with the study paradigm (Fig. 1).
Results
Five analyses were performed in order to examine limb-specific responses to emotional stimuli. The main effect of limb specificity was examined in order to verify that images of upper limbs would elicit greater activity in cervical spinal cord neurons than would images of lower limbs. The main effect of emotion was examined in order to demonstrate that our new stimulus set could replicate the emotional modulation of spinal cord neurons reported by Smith and Kornelsen (2011). Three specific condition contrasts were then performed in order to test the hypothesis that emotional stimuli will selectively enhance cervical spinal cord responses to emotional stimuli. In addition to the contrast maps displayed and described below, the time courses for the signal intensity data showed congruence with the stimulation paradigm (see Fig. 1).
Limb specificity (upper > lower)
Consistent with our first hypothesis, viewing images depicting upper-limb responses elicited greater levels of activity than did viewing images depicting lower-limb responses (see Fig. 2). In the contrast of upper-limb depictions of movement with lower-limb depictions of movement (i.e., upper-limb > lower-limb), 12 active voxels were detected in response to viewing an upper-limb image (p ≤ .0029); only four active voxels were detected in response to viewing a lower-limb image. As we noted in the Data Analysis section, each of these active voxels represents a statistically significant difference in signal intensity between the two conditions. These significant activations were detected throughout all four cervical spinal cord segments analyzed for the present study. The activity in C5/C6 appeared on the left side along the dorsal edge of the cord. Activity in C7/C8 appeared in ventral and medial areas of the left side of the cord. These results suggest that simply viewing an image implying movement elicits activity in the spinal cord regions related to the execution of this movement—in this case, of the upper limbs.
Emotional valence (negative > neutral)
Negative emotional images elicited greater levels of activity in motoric regions of the spinal cord than did neutral images (see Fig. 3). In the contrast of emotionally negative images and neutral images (negative > neutral), 27 active voxels were detected in response to viewing a negative image (p ≤ .0029); only two active voxels were detected in response to viewing a neutral image. The greatest concentration of activity was present in the right ventral and medial C6/C7 region, similar to the results reported by Smith and Kornelsen (2011). Segment C5 showed less activity, present only in the left dorsomedial cord.
Hand–negative > hand–neutral
One of the critical contrasts in the present study compared activity elicited by images depicting upper-limb responses to negative stimuli to activity elicited by images depicting upper-limb responses to neutral stimuli (see Fig. 4). In the contrast of negative upper-limb depictions and neutral upper-limb depictions (hand–negative > hand–neutral), 16 active voxels were detected in response to viewing a negative upper-limb image (p ≤ .0051); 11 active voxels were detected in response to viewing a neutral upper-limb image. The activity detected in the lower segments was concentrated on the right side of the cord, appearing in dorsomedial regions of C7/C8, and in ventral regions of C6/C7. Thus, consistent with our third hypothesis, images depicting hand responses to negative emotional stimuli elicited greater levels of activity than did images depicting hand responses to neutral stimuli.
At first glance, it would appear that the activity arising from both negative and neutral upper-limb movements elicited was less impressive than that seen in earlier analyses (e.g., 27 active voxels for the main effect of negative images). However, the 16 active voxels do not represent the absolute activity in these spinal cord segments; rather, they represent the contrast of the two conditions (i.e., the activity of the conditions relative to each other). This point is important, given that images depicting hand movement—even neutral hand movements—will elicit large amounts of activity in cervical spinal cord regions on the basis of the fact that nerves from these regions innervate the upper limbs. Therefore, the “baseline” level of activity represented by the neutral upper-limb condition is higher than it would be for the lower-limb conditions described below, leaving only the effect of emotion to differentiate between the two upper-limb conditions in this contrast.
Hand–negative > foot–negative
This contrast tested whether the effect of emotion was greater for images related to the upper limbs, the area of the body innervated by the cervical spinal cord (see Fig. 5). In the contrast of negative upper-limb depictions and negative lower-limb depictions (hand–negative > foot–negative), 31 active voxels were detected in response to viewing a negative upper-limb image (p ≤ .0051), whereas ten active voxels were detected in response to viewing a negative lower-limb image. Specifically, segments C7/C8 showed primarily left ventral and dorsal activity. Dorsomedial activity was detected in the right side of segment C5. The activity in segments C6/C7 was primarily ventral and on the right side of the cord. Therefore, consistent with our fourth hypothesis, negative-emotion images depicting upper-limb responses evoked more activity than did negative images depicting lower-limb responses, suggesting that the effect of emotion was limb-specific.
Foot–negative > foot–neutral
An additional single-condition planned comparison was performed to contrast activity between negative lower-limb depictions (foot–negative) and neutral lower-limb depictions (foot–neutral) runs. It is important to note that the total amount of activity was greater overall for conditions involving the upper rather than the lower limbs. However, within this smaller pool of activity, the contrast for foot–negative > foot–neutral indicated substantially more activity in the foot–negative condition (35 active voxels) than in the foot–neutral condition (17 active voxels; p ≤ .0051). This suggests that emotional valence had a larger influence on activity for lower- than for upper-limb conditions. Although seemingly counterintuitive, the reason for this difference was that the hand–neutral images elicited a great deal of neuronal activity overall. Therefore, as discussed above, the “baseline” (neutral condition) for the hand–negative > hand–neutral contrast would be larger, thus reducing the difference between hand–negative and hand–neutral conditions. In contrast, because the lower limbs are not innervated by the spinal cord segments targeted in this study, the “baseline” (neutral condition) for the foot–negative > foot–neutral contrast would be relatively low. Therefore, any effect of emotion for lower-limb conditions would appear quite pronounced.
When we also consider that the hand–negative condition resulted in more activity than the foot–negative condition, these data suggest that negative emotional images elicit two effects on spinal cord neurons: (1) a general “alarm” signal that is not limb-specific (as seen in the foot–negative > foot–neutral contrast), and (2) an additional limb-specific response facilitating a situation-appropriate movement (as seen in the hand–negative > foot–negative activity).
Discussion
The present study demonstrates that emotional stimuli modulate motoric responses in specific, response-appropriate ways. The fact that negative images of the upper limbs elicited greater activity than did neutral images of the upper limbs demonstrates that emotion influences the functioning of neurons in the cervical spinal cord. The finding that negative images of the upper limbs (which are innervated by the cervical spinal cord) elicit greater activity than do negative images of the lower limbs (which are not innervated by the cervical spinal cord) suggests that this modulation reflects a specific emotional movement such as raising one’s arms to protect one’s face. The neuronal responses occurred in the lower cervical spinal cord segments, as in our previous research (Smith & Kornelsen, 2011). This region of the spinal cord innervates the hands, the portion of the upper limbs most likely to be used for precise motoric behaviors.
Within the cross-section of the spinal cord, much of the activity occurred in ventral and dorsomedial regions. The ventral region of the cord modulates the efferent spinal nerves involved with the initiation of movements. Dorsomedial voxels may reflect the activity of interneurons, whose functions include communication within and between segments of the spinal cord. It is possible that this activity represents descending modulation of movement. During the execution of movements, activity from the brain does not simply connect monosynaptically with the correct spinal cord segment motor neurons in order to trigger a response. Instead, this input is relayed through interneurons, which project to other interneurons and to motor neurons (Purves et al., 2008). The result is that descending input from the brain can both stimulate movements and inhibit the contraction of other muscles, thus allowing for a precise motoric plan to be executed. Therefore, the dorsomedial activity found in the present study may represent the fine-tuning of the motoric plans that would be appropriate in response to each stimulus. If this is the case, it would be the first spinal fMRI demonstration that simply viewing a pictorial depiction of a limb can trigger neuronal responses similar to those involved in performing the action.
Overall, our results are consistent with the view that an emo-motoric network involves both the brain and spinal cord (Smith & Kornelsen, 2011). Numerous studies have noted increases in responsivity in motor regions of the brain that transmit information to the spinal cord (Oliveri et al., 2003; Schienle et al., 2005). Previous research has also noted a relationship between activity in the amygdala, a limbic region activated in response to emotionally arousing stimuli, and the supplementary motor cortex (Sagaspe et al., 2011; Tettamanti et al., 2012). Given that several TMS studies have revealed increased motor-evoked potentials in response to emotional versus neutral stimuli (Coelho et al., 2010; Coombes et al., 2009; Hajcak et al., 2007; Schutter et al., 2008; van Loon et al., 2010), suggestive of increased corticospinal tract excitability, it seems reasonable to assume that our results reflect an amygdala-based modulation of motoric programming. Future research could address this issue by assessing spinal cord responses in clinical populations with amygdala damage or dysfunction (e.g., temporal lobe epilepsy, autism, or PTSD).
Although the present exploratory study extends our understanding of the emotional modulation of movement, some limitations must also be addressed in future experiments. One key issue is the need for stimuli that depict only the left or the right hand. This exploratory study included images depicting left, right, and bilateral limb use; this likely contributed to the variability in the precise locations of the active voxels (i.e., activity appeared on both sides of the cord rather than being isolated to the right side). Having now established that visual depictions of movement can elicit spinal cord responses, future research with more homogeneous stimuli will allow us to examine more specific issues, such as whether the hand depicted in the image can elicit lateralized spinal cord activity. Furthermore, including the use of MR-compatible electromyography in future studies would reinforce the observed effects by eliminating the concern that the increased responsiveness of the ventral motor neuron pool was inflated by muscle tensing of the upper limbs. Additionally, acquiring physiological measures for inclusion as confounds in the general linear model would have allowed us to reduce noise and motion artifacts due to heart rate and respiration (Kong, Jenkinson, Andersson, Tracey, & Brooks, 2012). Finally, while the present study demonstrates the existence of an emo-motoric pathway at the cervical level, confirming the presence of such a pathway in the lumbar cord by eliciting more activity in lower-limb than in upper-limb conditions (specifically, foot–negative > foot–neutral and foot–negative > hand–negative) would more concretely establish the specialization of such a system. Future studies will address this issue.
The present study suggests that cervical spinal cord neurons not only process negative emotional stimuli via the previously identified general “alarm” system (Smith & Kornelsen, 2011), but also through a more refined, limb-specific response. These findings are consistent with an “action preparedness” model of emotion, whereby emotional stimuli elicit activity in nervous system structures that could, if necessary, initiate a situation-specific response (Schutter et al., 2008; van Loon et al., 2010). The present research therefore extends our knowledge of emotion–movement interactions. Future research will allow us to further delineate the specific neural mechanisms underlying this emotional modulation of spinal cord activity.
References
Coelho, C. M., Lipp, O. V., Marinovic, W., Wallis, G., & Riek, S. (2010). Increased corticospinal excitability induced by unpleasant visual stimuli. Neuroscience Letters, 48, 135–138. doi:10.1016/j.neulet.2010.03.027
Coombes, S. A., Tandonnet, C., Fujiyama, H., Janelle, C. M., Cauraugh, J. H., & Summers, J. J. (2009). Emotion and motor preparation: A transcranial magnetic stimulation study of corticospinal motor tract excitability. Cognitive, Affective, & Behavioral Neuroscience, 9, 380–388. doi:10.3758/CABN.9.4.380
Davidson, R. J., & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3, 11–21. doi:10.1016/S0028-3932(98)00089-X
Hajcak, G., Molnar, C., George, M. S., Bolger, K., Koola, J., & Nahas, Z. (2007). Emotion facilitates action: A transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology, 44, 91–97. doi:10.1111/j.1469-8986.2006.00487.x
Kiernan, J. A. (1998). The human nervous system: An anatomical viewpoint (7th ed.). Philadelphia, PA: Lippincott-Raven.
Koganemaru, S., Domen, K., Fukuyama, H., & Mima, T. (2012). Negative emotion can enhance human motor cortical plasticity. European Journal of Neuroscience, 35, 1637–1645. doi:10.1111/j.1460-9568.2012.08098.x
Kong, Y., Jenkinson, M., Andersson, J., Tracey, I., & Brooks, C. W. (2012). Assessment of physiological noise modelling methods for functional imaging of the spinal cord. NeuroImage, 60, 1538–1549. doi:10.1016/j.neuroimage.2011.11.077
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International Affective Picture System (IAPS): Technical manual and affective ratings (Technical Report No. A-4). Gainesville, FL: University of Florida, Center for Research in Psychophysiology.
Oliveri, M., Babiloni, C., Filippi, M. M., Caltagirone, C., Babiloni, F., Cicinelli, P., et al. (2003). Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Experimental Brain Research, 149, 214–221. doi:10.1007/s00221-002-1346-8
Pereira, M. G., de Oliveira, L., Erthal, F. S., Joffily, M., Mocaiber, I. F., Volchan, E., et al. (2010). Emotion affects action: Midcingulate cortex as a pivotal node of interaction between negative emotion and motor signals. Cognitive, Affective, & Behavioral Neuroscience, 10, 94–106. doi:10.3758/CABN.10.1.94
Purves, D., Augustine, G. J., Fitzpatrick, D., Hall, W. C., LaMantia, A., McNamara, J. O., et al. (2008). Neuroscience (4th ed.). Sunderland, MA: Sinauer.
Sagaspe, P., Schwartz, S., & Vuilleumier, P. (2011). Fear and stop: A role for the amygdala in motor inhibition by emotional signals. NeuroImage, 55, 1825–1835. doi:10.1016/j.neuroimage.2011.01.027
Schienle, A., Schäfer, A., Walter, B., Stark, R., & Vaitl, D. (2005). Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neuroscience Letters, 388, 1–6. doi:10.1016/j.neurlet.2005.06.025
Schutter, D. J., Hofman, D., & Van Honk, J. (2008). Fearful faces selectively increase corticospinal motor tract excitability: a transcranial magnetic stimulation study. Psychophysiology, 45, 345–348. doi:10.1111/j.1469-8986-2007.00635.x
Smith, S. D., & Kornelsen, J. (2011). Emotion-dependent responses in spinal cord neurons: A spinal fMRI study. NeuroImage, 58, 269–274. doi:10.1016/j.neuroimage.2011.06.004
Stroman, P. W. (2005). Magnetic resonance imaging of neuronal function in the spinal cord: spinal FMRI. Clinical Medicine & Research, 3, 146–156. doi:10.3121/cmr.3.3.146
Stroman, P. W., Bosma, R. L., & Tsyben, A. (2012). Somatotopic arrangement of thermal sensory regions in healthy human spinal cord determined by means of spinal cord functional MRI. Magnetic Resonance in Medicine, 68, 923–931. doi:10.1002/mrm.23292
Tettamanti, M., Rognoni, E., Cafiero, R., Costa, T., Galati, D., & Perani, D. (2012). Distinct pathways of neural coupling for different basic emotions. NeuroImage, 59, 1804–1817. doi:10.1016/j.neuroimage.2011.08.018
van Loon, A. M., van den Wildenberg, W. P., van Stegeren, A. H., Hajcak, G., & Ridderinkhof, K. R. (2010). Emotional stimuli modulate readiness for action: A transcranial magnetic stimulation study. Cognitive, Affective, & Behavioral Neuroscience, 10, 174–181. doi:10.3758/CABN.10.2.174
Author note
This research was supported by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McIver, T.A., Kornelsen, J. & Smith, S.D. Limb-specific emotional modulation of cervical spinal cord neurons. Cogn Affect Behav Neurosci 13, 464–472 (2013). https://doi.org/10.3758/s13415-013-0154-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0154-x