Abstract
The aim of the present study was to examine whether interindividual differences in the coupling or decoupling of prefrontal and posterior cortices during the exposure to social–emotional information may predict an individual’s positive emotional responsiveness. Susceptibility to humor was assessed in a behavioral paradigm several weeks after the EEG recordings. State-dependent changes of prefrontal–posterior EEG beta coherence were recorded during stimulation with other people’s auditory expressions of cheerfulness and sadness. Greater decreases of coherence during the stimulation with positive affect expressions prospectively predicted greater positive emotional responsiveness, indicated by higher amusement ratings in response to cartoons and higher scores in a questionnaire measure of exhilarability. Greater increases of coherence during the stimulation with negative affect expressions did not predict perceived funniness but were related to shorter response latencies to the amusement ratings. The results further support the notion that a more loose prefrontal–posterior coupling may be related to loosening of control of the prefrontal cortex over incoming emotional information and, thus, to a propensity to deeper emotional involvement and a greater impact of perceptual input, whereas increased prefrontal–posterior coupling may be related to strong control and the propensity to protect oneself from becoming emotionally affected.
Similar content being viewed by others
Evidence suggests that remote brain regions may influence perceptual processing and awareness mediated by posterior sensory and association cortices and that the functional connectivity between cortical regions is modulated in support of dynamically changing processing demands (Sepulcre et al., 2010; Vuilleumier & Driver, 2007). However, little is known to date about the significance of state-dependent changes in cortico-cortical connectivity in the context of affective processing, which may play an important role besides the connections between cortical and subcortical structures (above all, the amygdala; (Davidson, 2002; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Phillips, Ladouceur, & Drevets, 2008).
Current models of potential mechanisms involved in the processing of social–emotional information assume the contribution of both a bottom-up and a top-down component. The bottom-up process, which is automatically activated by perceptual input, is supposed to be modulated in a top-down fashion through an executive control component implemented in the prefrontal cortex (see Decety & Moriguchi, 2007, for a review). Neuroscientific studies confirmed that the prefrontal cortex exerts feedback control on posterior association cortices, in order to further modulate representations of affectively relevant information. For instance, during the exposure to highly emotionally arousing (threatening) pictures, EEG coherence between the prefrontal and the posterior association cortices increased, as compared with neutral images, which was interpreted as activation of a top-down mechanism related to rejection of the negative content or downregulation of negative affect (Miskovic & Schmidt, 2010; see also Schellberg, Besthorn, Klos, & Gasser, 1990). Increases of EEG coherence are considered to indicate increased connectivity and functional communication between two neuronal populations (Fries, 2005; Srinivasan, Winter, Ding, & Nunez, 2007). That is, state-dependent changes of prefrontal–posterior EEG coherence may reveal relevant coupling and decoupling of cortical networks related to regulatory processes in the context of affective processing. The EEG findings are corroborated by data from a magnetic resonance imaging study suggesting that anticipatory mental imagery of a mildly fearful facial emotional expression proactively altered the subjective experience of highly fearful faces by state-dependent top-down regulatory influences of the prefrontal cortex on the temporoparietal cortex (Diekhof et al., 2011).
In a recent study, we were able to show that more loose prefrontal–posterior coupling may be related to loosening of control of the prefrontal cortex over incoming social–emotional information and, consequently, to a propensity to deeper emotional involvement, whereas, by contrast, increased prefrontal–posterior coupling during social–emotional stimulation may be related to strong control, dampening of emotional experience, and the propensity to protect oneself from becoming emotionally affected (Reiser et al., 2012). Taken together, interindividual differences in state-dependent changes of EEG coherence between prefrontal and posterior cortical regions may be indicative of a mechanism modulating the impact social–emotional information has on the individual and which, therefore, may make individuals either more or less dependent on external emotional cues.
In order to further examine the significance of interindividual differences in the proposed top-down regulatory mechanism during the exposure to social–emotional information, the present study was designed to examine their role in the context of an individual’s susceptibility to positive affect. Independently from dispositional factors related to negative affect, a disposition to greater positive emotional responsivity could be relevant, because it may, for instance, foster resilience against and recovery from affective disorders (Catalino & Fredrickson, 2011; Rottenberg, Kasch, Gross, & Gotlib, 2002). The experience of positive emotions, especially amid challenge and adversity, contributes to successful adaptation to challenges—for instance, to those of later life (Ong, Bergeman, Bisconti, & Wallace, 2006). Humor serves important cognitive and emotional functions in these processes. Therefore, in the present study, interindividual differences in the (de-)coupling of prefrontal and posterior cortical regions during social–emotional stimulation were related to interindividual differences in positive emotional responsivity—specifically, in the susceptibility to humor. Previous research suggested that the correlates of individual differences in prefrontal–posterior coupling or decoupling can be emotion specific (Reiser et al., 2012). Consequently, the present study also examined whether the coherence changes during the social–emotional stimulation with positive versus negative affect expressions may be differentially valid for the prediction of interindividual differences in humor susceptibility. To this end, state-dependent changes of EEG coherence were recorded during the exposure to other people’s expressions of cheerfulness and sadness.
Humor is primarily a social–emotional phenomenon, and its occurrence in the absence of other persons is considered pseudosocial in nature (Martin, 2007). To appreciate humor, it is required to perceive a situation or event in two habitually incompatible associative contexts. The perception and the resolution of the perceived incongruity result in amusement, which is a pleasurable experience (Ruch, 2001; Suls, 1972). In addition to basic flexibility and openness (Shammi & Stuss, 2003; Weiss et al., in press), empathizing or mentalizing is required to appreciate most forms of humor (Samson, 2012; Samson, Zysset, & Huber, 2008).
There is some evidence suggesting that interindividual differences in the functional connectivity between prefrontal and posterior cortical regions might have an impact on humor susceptibility. For instance, in accordance with their propensity to a more flexible or loose associative processing (Gianotti, Mohr, Pizzagalli, Lehmann, & Brugger, 2001; Mohr, Graves, Gianotti, Pizzagalli, & Brugger, 2001; Rominger, Weiss, Fink, Schulter, & Papousek, 2011), students scoring higher on positive schizotypal symptoms (in the subclinical range) scored higher on a measure of humor appreciation (experienced funniness of jokes) than those with lower schizotypy scores. Congruously, in individuals with high schizotypal personality scores, as well as in patients with positive schizophrenic symptoms, decreased functional connectivity between prefrontal and posterior cortices was observed (Higashima et al., 2007; Koychev, Deakin, Haenschel, & El-Deredy, 2011; Lawrie et al., 2002; Nakamura et al., 2005; Vercammen, Knegtering, den Boer, Liemburg, & Aleman, 2010; Winterer, Coppola, Egan, Goldberg, & Weinberger, 2003). In addition, researchers have linked an activation of temporoparietal areas during stimulation with humorous material to mentalizing, assuming that better mentalizing abilities are related to increased experienced funniness of humor stimuli (for a review, see Kohn, Kellermann, Gur, Schneider, & Habel, 2011). Thus, less top-down inhibition of these temporoparietal areas by the prefrontal cortex should make an individual more susceptible to other people’s expressions of amusement, as well as more susceptible to humor.
In sum, the study aimed at adding to the scarce literature on the functional significance of state-dependent changes in cortico-cortical connectivity in the context of affective processing by demonstrating their relevance to an individual disposition that is assumed to affect the recovery from and protection against affective disturbances. Interindividual differences in prefrontal–posterior EEG coherence in the context of affective processing qualify as a neurophysiological correlate or substrate of affect-related dispositions or disorders only if they can predict relevant variables assessed in some temporal distance from the EEG recordings. Therefore, in the present study, the participants’ susceptibility to humor was assessed several weeks after the EEG recordings. Humor susceptibility was assessed by a behavioral paradigm in which the participants rated nonverbal cartoons for funniness. In addition to the behavioral measures, a questionnaire assessing trait cheerfulness was applied, which is supposed to form the temperamental basis of interindividual differences in positive emotional reactivity (“exhilarability”; Ruch & Köhler, 2007). It was expected that greater decreases/smaller increases in EEG coherence during the social–emotional stimulation, particularly during the exposure to others’ expressions of cheerfulness, would predict greater susceptibility to humor.
Method
Participants
Thirty-eight right-handed university students 18–36 years of age completed the experiment (M = 23.5, SD = 4.4; 18 women, 20 men). Handedness was assessed by a standardized handedness test (performance test; Papousek & Schulter, 1999; Steingrüber & Lienert, 1971). Participants were requested to refrain from alcohol for 12 h and from coffee and other stimulating beverages for 4 h prior to their lab appointment and to come to the session well rested. No participant reported using drugs or psychoactive medication, and none had participated in an experiment using cartoons before. EEG data were statistically analyzed only if at least 30 s of artifact-free data were available in each of the included electrode positions (see below). This resulted in a reduction of the sample to n = 32 (cheerfulness stimulation; 15 women, 17 men) and n = 31 (sadness stimulation; 13 women, 18 men). The study was performed in accordance with the 1964 Declaration of Helsinki and was approved by the local ethics committee. Participants gave their written consent to participate.
Materials
Social–emotional stimulation
Three clips from the set of “Emotionally Contagious Sound Clips” (ECOS; Papousek, Reiser, Weber, Freudenthaler, & Schulter, 2012; Weber, Papousek, & Schulter, 2011) were used: ECOS–C (cheerfulness; hearty laughter), ECOS–S (sadness; bitter weeping and sobbing), and ECOS–N, serving as the reference condition (neutral; soft murmurs and trivial everyday sounds without understandable language). In each of the sound clips (90 s each), a small mixed-gender group of people audibly expresses the respective affect without using language (i.e., words or parts of words). The clips were matched for peak sound intensity and sound level range and were presented over headphones. Previous studies had confirmed the effectiveness of the sound clips to evoke the respective affective states in the listener. Since very strong emotions are displayed, healthy participants have no difficulties identifying and differentiating the expressed affective states (Papousek, Freudenthaler, & Schulter, 2011; Papousek et al., 2012, ; Reiser et al., 2012). Affect ratings were used to check whether the sound clips evoked the respective affective states. The participants were instructed as follows: Please indicate what effect the sound clip had on you personally: “The sound clip affected me with cheerfulness (sadness).” Ratings included two further emotions (anxiety, anger). Participants indicated their judgments on 10-cm horizontal visual analogue scales presented on the computer screen. The responses were scored in millimeters from 0 (do not agree at all) to 100 (strongly agree).
Humor paradigm
The stimulus material consisted of three types of cartoons, as well as nonhumorous pictures serving as a control condition. They were used in previous studies (e.g., Samson & Hegenloh, 2010; Samson, Lackner, Weiss, & Papousek, 2012; Samson et al., 2008). Not directly relevant to the research question addressed in the present study, the three types of cartoons were not analyzed separately. The nonhumorous control pictures were cartoon-like pictures containing an incongruity that could not be resolved meaningfully; that is, they did not contain a punch line. All pictures were low in aggressive, violent, and sexual content.Footnote 1 During the experiment, a total of 96 pictures were presented, divided into three blocks of 32 pictures each. Within each block, the pictures (24 cartoons and 8 nonhumorous pictures) were presented in random order. A 10-min break was provided between the blocks. The order of blocks was counterbalanced. During the behavioral humor paradigm, the participants rated each cartoon for how funny they perceived it to be (amusement rating; from 1 = not funny, to 6 = very funny). The validity of this rating has been confirmed by a study showing that higher amusement ratings were intra- and interindividually correlated with larger heart rate responses to the detection of the punch line in cartoons (Lackner et al., 2012). The computer program also calculated the response latencies to the amusement ratings (starting from the onset of the rating scale).
Questionnaires
As a questionnaire measure of interindividual differences in “exhilarability,” the trait form of the State-Trait-Cheerfulness Inventory (STCI) was applied (Ruch, Köhler, & van Thriel, 1996). It contains 60 items rated from 1 (strongly disagree) to 4 (strongly agree). High total scores on the STCI indicate high exhilarability—that is, a high readiness to respond to a humorous stimulus with positive affect (Ruch & Köhler, 2007; Ruch et al., 1996).
Since it seemed plausible that depressed mood could affect the participants’ susceptibility to humor, and to be able to genuinely attribute the effects to positive emotional responsivity (excluding the possibility that they may merely be due to low levels of negative affect), depression was controlled using the Center for Epidemiologic Studies Depression Scale (CES–D; German version: Hautzinger & Bailer, 1993). It consists of 20 items referring to mood and attributions over the past week. It is particularly suitable to measuring subclinical depressive experiences in the general population (Wood, Taylor, & Joseph, 2010). In addition, as an indicator of their motivation, the participants were asked to indicate after each block of the humor paradigm how much effort they had made to accomplish the task (17-point rating scale, from 1 = not at all, to 17 = extremely). Previous findings showing higher amusement ratings in participants having expended more efforts indicated that motivation/efforts may influence the behavioral data in this paradigm (Samson et al., 2012).
Procedure
Data were obtained in two separate sessions. In the first session, the EEG was recorded during auditory social–emotional stimulation. In the second session, the humor paradigm was applied. The two sessions were separated by 4–6 months.
After performance of the hand dominance test, participants were seated in an acoustically and electrically shielded examination chamber, and electrodes were attached. The participants were instructed via headphones that in each of the following short sound clips, they would hear a group of people; they were instructed to close their eyes, direct their whole attention to what they would hear, and imagine that they were part of the group. The EEG was recorded during each sound clip. Emotionally arousing and neutral sound clips were presented alternating, so that each emotional sound (ECOS–S or ECOS–C) was preceded and followed by ECOS–N (e.g., N–S–N–C–N). After each sound clip, the participants answered the affect ratings, using the computer mouse with their preferred (i.e., right) hand. The order of emotional sounds was counterbalanced.
In the second session, the participants were tested in the same examination room. After the CES–D and the STCI were administered, the participants received instructions for the task and were given sample cartoons and the required responses. Each cartoon was presented for 6 s on a computer screen, along with two buttons at the bottom of the screen (“not understood,” “understood”). Participants were required to indicate via mouse click whether they had or had not understood the punch line of the cartoon. After presentation of each cartoon, a scale appeared for 4 s on which the participants rated via mouse click the funniness of each cartoon (amusement rating).
In both sessions, the technical equipment and the experimenter were located outside the chamber, and the participants were monitored through a one-way window and an intercom.
EEG recording and quantification
EEG was recorded from 19 channels according to the international 10–20 system, using a Brainvision BrainAmp Research Amplifier (Brain Products; sampling rate, 500 Hz; resolution, 0.1 μV) and a stretchable electrode cap, referenced to the nose and rereferenced offline to a mathematically averaged ears reference (Essl & Rappelsberger, 1998; Hagemann, 2004). Impedance was kept below 5 kΩ for all electrodes. Horizontal and vertical EOG measures were obtained for identification of ocular artifacts. All data were inspected visually, in order to eliminate intervals in which ocular or muscle artifacts occurred. Artifact-free EEG data were submitted to fast Fourier analysis using a Hanning window (epoch length, 1 s; overlapping, 10%; low-cut filter, 0.016 Hz). Spectral coherence (Fisher’s z-transformed) was obtained in the beta band (13–30 Hz) using the quotient of the cross spectrum and the auto spectra (power spectra) according to the following equation: \( {{{Coh\left( {{c_1},{c_2}} \right)(f)={{{\left| {CS\left( {{c_1},{c_2}} \right)(f)} \right|}}^2}}} \left/ {{(\left| {CS\left( {{c_1},{c_1}} \right)(f)} \right|\times \left| {CS\left( {{c_2},{c_2}} \right)(f)} \right|),\ \mathrm{with}\,CS\left( {{c_1},{c_2}} \right)(f)=\sum {c_{1,i }}(f){c_{2,i }}(f).Coh\left( {{c_1},{c_2}} \right)(f)}} \right.} \) denotes the coherence at frequency f between electrodes 1 and 2, which can vary between 0 and 1.
Previous research on EEG coherence in the context of affective processing indicated that connectivity changes during evoked emotions occurred primarily in the beta frequency range (Aftanas, Lotova, Koshkarov, & Popov, 1998; Miskovic & Schmidt, 2010; Reiser et al., 2012; Schellberg et al., 1990). Research also suggested that beta-band oscillations are particularly important for mediating long distance coupling (Gross et al., 2004; Kopell, Ermentrout, Whittington, & Traub, 2000; Schnitzler & Gross, 2005). Consequently, in the present study, we focused on coherence in the beta frequency range (13–30 Hz). In order to avoid an unnecessary great number of statistical comparisons, we do not report other frequency bands.
Following Miskovic and Schmidt (2010) and our previous research (Reiser et al., 2012), coherence pairs were grouped into anatomically valid clusters corresponding to the left and right, prefrontal and posterior association cortex regions. Coherence scores of nine electrode pairs each were averaged to summarize interaction within the left and the right hemispheres, respectively (left: Fp1–T3, Fp1–P3, Fp1–T5, F3–T3, F3–P3, F3–T5, F7–T3, F7–P3, F7–T5; right: Fp2–T4, Fp2–P4, Fp2–T6, F4–T4, F4–P4, F4–T6, F8–T4, F8–P4, F8–T6). By using these clusters, we avoided a hardly manageable inflation of the number of statistical tests. To ensure reliability, data were further analyzed only if at least 30 s of artifact-free data were available for a condition in each of the included electrode positions.
Data analysis
Linear regressions were conducted using the EEG beta coherence during the neutral (reference) sound preceding the emotionally arousing sound to predict the coherence during listening to the emotionally arousing sound, in order to calculate residualized change scores, which were used as an index of state-dependent decreases or increases of intrahemispheric coherence in response to the emotional provocation. This was done to ensure that the analyzed residual variability was due to the experimental manipulation, and not to individual differences in baseline levels, and to control for measurement error inherent in the use of repeated measures of the same kind (e.g., Linden, Earle, Gerin, & Christenfeld, 1997; Steketee & Chambless, 1992). In the following, the abbreviation “Δcoh” will be used for these change-of-coherence scores. Negative scores indicate a decrease in prefrontal–posterior coherence; positive scores indicate an increase.
Data from not understood cartoons and “understood” control pictures and trials on which participants failed to answer the amusement rating were excluded from further analysis. On average, M = 66.4 (SD = 5.9); that is, 92% of the punch lines were understood. The amusement ratings (internal consistency reliability, α = .95) and response latencies to the amusement ratings (α = .93) were averaged across all items of all blocks. Residualized scores were calculated, in order to control for general individual differences in response tendencies and response speed (e.g., Fazio, 1990). This was done by conducting linear regressions using the scores of the control pictures to predict those of the cartoons (see also Samson et al., 2012). The use of residualized scores ensured that the analyzed residual variability was specific to the participants’ evaluation of the experience of humor and accompanying feelings of amusement and exhilaration, as opposed to the evaluation of any pictures or recognition of any incongruence. (The answers to the comprehensibility questions were not analyzed, because there was only very little variance in them).
To evaluate whether interindividual differences in the coherence changes during the social–emotional provocation would predict the participants’ susceptibility to humor in the behavioral paradigm, hierarchical multiple regression analyses were performed, with the amusement rating or the response latency to the amusement rating as the dependent variable. In step 1, the depression and the efforts scores were entered. The coherence scores (Δcoh) for the cheerfulness sound or for the sadness sound were entered in step 2. A significant F change test indicates that Δcoh explained a significant amount of variance of how funny the participants perceived the cartoons to be or how long it took them to deliver the ratings, independently of potential influences of depression or effort. R 2 change indicates the amount of variance the coherence changes could explain independently of variance that might be explained by depression or efforts. Since sex did not explain any variance of state-dependent EEG beta coherence changes during exposure to social–emotional stimulation in previous experiments (Reiser et al., 2012), it was not included as a predictor. Since previous research suggested lateralized effects of prefrontal–posterior coupling in the context of affective processing (Reiser et al., 2012; Schellberg et al., 1990), coherence changes were analyzed separately for the left and the right hemispheres.
To test whether interindividual differences in the coherence changes during the social–emotional provocation may predict the participants’ “exhilarability” as assessed by the STCI, analogous hierarchical multiple regression analyses were performed, with depression scores entered in step 1 and Δcoh for the cheerfulness stimulation or for the sadness stimulation in step 2. To confirm the effectiveness of the emotional provocation, a multivariate analysis of variance with sound clip (ECOS–N, ECOS–C, ECOS–S; within-subjects factor) as the independent variable and subjective affect ratings as the dependent variable was performed. Ratings of ECOS–N preceding ECOS–C and ECOS–S were averaged for this analysis.
Results
Coherence changes in response to the cheerfulness sound clip
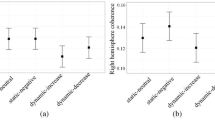
Coherence changes (Δcoh) in the right hemisphere in response to the stimulation with laughter predicted how funny the cartoons were perceived to be, F change (1, 28) = 4.7, p < .05; β = −.35, R 2 change = .12. A decrease of prefrontal–posterior coherence was associated with higher amusement ratings (Fig. 1). Efforts was also a significant predictor of perceived funniness, higher efforts to accomplish the task being associated with higher amusement ratings (β = .51, p < .01). The contribution of depression was not significant (β = −.30, n.s.). By contrast, Δcoh during the cheerfulness stimulation did not predict the response latencies to the amusement ratings, F change (1, 28) = 1.1, n.s. The analogous analyses with Δcoh in the left hemisphere yielded no significant results, F change (1, 28) = 0.2, n.s.; F change (1, 28) = 0.2, n.s.; F change (1, 28) = 0.5, n.s.. Changes of EEG beta coherence (Δcoh) in the right hemisphere also predicted trait exhilarability as assessed by the STCI, F change (1, 29) = 4.4, p < .05; β = −.34, R 2 change = .11; left hemisphere: F change (1, 29) = 0.0, n.s. Greater prefrontal–posterior decoupling during exposure to the cheerfulness sound clip was associated with higher STCI scores.Footnote 2
Prediction of amusement ratings in the humor paradigm by changes of prefrontal–posterior EEG coherence (Δcoh) from neutral stimulation to stimulation with expressions of cheerfulness (right hemisphere). Adjusted for depression and efforts. The plot shows standardized residuals (see the Data Analysis section)
Coherence changes in response to the sadness sound clip
The analysis of coherence changes in response to the sadness sound clip did not predict how funny the cartoons were rated as being, F change (1, 27) = 0.1, n.s. However, EEG data of the sadness stimulation showed that Δcoh in the right hemisphere predicted the response latencies to the amusement ratings, F change (1, 27) = 7.8, p < .01; β = −.48, R 2 change = .22. Participants showing greater increases of prefrontal–posterior coherence in response to the sadness stimulation took less time to respond to the amusement ratings (Fig. 2). The analogous analyses with Δcoh in the left hemisphere yielded no significant results for funniness but a significant result for the response latencies to the amusement ratings, F change (1, 27) = .0, n.s.; F change (1, 27) = 1.0, n.s.; F change (1, 27) = 4.2, p = .05, β = −.38, R 2 change = .13. No associations were found between Δcoh in response to the provocation with sadness and the scores on the STCI, F change (1, 28) = 0.1, n.s.; F change (1, 28) = 1.3, n.s.2.
Prediction of response latencies to the amusement ratings in the humor paradigm by changes of prefrontal–posterior EEG coherence (Δcoh) from neutral stimulation to stimulation with expressions of sadness (right hemisphere). Adjusted for depression and efforts. The plot shows standardized residuals (see the Data Analysis section)
Supplemental analyses
EEG coherence changes between the emotional and the neutral conditions ranged from −.04 to .04. The respective multivariate analysis of variance confirmed the effectiveness of the emotional provocation, F(4, 146) = 107.1, p < .001; cheerfulness, F(2, 74) = 174.7, p < .001, η p 2 = .83; sadness, F(2, 74) = 151.5, p < .001, η p 2 = .80. Means of the cheerfulness rating were M = 33.6 (SD = 22.9), M = 88.4 (SD = 19.8), and M = 8.7 (SD = 11.19) for ECOS–N, ECOS–C, and ECOS–S, respectively. Means of the sadness rating were M = 12.8 (SD = 16.9), M = 6.2 (SD = 10.0), and M = 72.0 (SD = 27.4). The correlations between the coherence changes (Δcoh) and the respective emotion ratings after the ECOS induction were in the expected direction but failed to be statistically significant (ECOS–C, righthemisphere r = −.26, left r = −.16; ECOS–S, right r = −.23, left r = −.20).
Participants rated the cartoons (M = 3.7, SD = 0.6) more funny than the control pictures (M = 1.2, SD = 0.3), t(37) = 23.5, p < .001. The ratings of funniness (residualized scores) were not significantly correlated with the response latencies to the amusement ratings (r = −.23, n.s.).
In order to test whether the coherence changes might have been influenced by changes in enhanced myogenic activity, we additionally calculated correlations between the coherence changes (Δcoh ECOS–C, ECOS–S, left, right) and the respective changes of the spectral power in the range of 65–75 Hz, which is presumed to be exclusively myogenic in origin (averaged across the used electrode positions). These correlations ranged from r = .11 (p = .57) to r = .18 (p = .33).
Discussion
Previous evidence suggested that state-dependent changes of prefrontal–posterior EEG coherence may be indicative of a mechanism modulating the impact of emotional information on the individual. The present study provides additional evidence for the significance of interindividual differences in the functional coupling or decoupling of prefrontal and posterior cortical regions in the context of affective processing. Specifically, interindividual differences in state-dependent EEG beta coherence during exposure to other people’s expressions of cheerfulness prospectively predicted an individual’s susceptibility to humor. Participants showing greater state-dependent decreases of coherence during the social–emotional stimulation with cheerful affect expressions rated cartoons as being more funny than did participants in whom the prefrontal–posterior coherence had decreased to a lesser extent or had increased. This finding is in line with previous results showing that a more loose prefrontal–posterior coupling may be related to a propensity to deeper emotional involvement and a greater impact of perceptual input. By contrast, increased prefrontal–posterior coupling may be related to stronger control and the propensity to protect oneself from becoming emotionally affected (Reiser et al., 2012). This is additionally supported by the finding of a correlation of greater decreases of coherence during the cheerfulness stimulation, with a greater self-reported positive emotional responsivity as a trait. Together, these findings add to the evidence of validity of the observed EEG coherence changes and indicate that the proposed top-down modulating mechanism is relevant in the context of individual differences related to positive affect.
Remarkably, interindividual differences in the susceptibility to humor and positive emotional responsiveness were predicted by individual differences in prefrontal–posterior (de-)coupling only during exposure to positive, but not negative, affect expressions. This suggests that the functional connectivity between cortical regions may be modulated depending on the particular emotion that is perceived. However, whereas the EEG coherence changes during the sadness stimulation did not predict the perceived funniness of the cartoons, they were related to the response latencies to the amusement ratings. Participants took less time to deliver the amusement ratings if they were showing greater increases of prefrontal–posterior coherence in response to the sadness stimulation. The response latencies for the amusement ratings are related to the easiness of perceiving and judging one’s amusement (Samson et al., 2012). There is some evidence that this implicates effective regulation of negative emotions. During the viewing of cartoons, negative feelings also may arise to a certain extent, because most jokes (also those that are not aggressive) involve a victim (the butt of a joke) who, for instance, has a false belief. This is associated with negative emotions that the viewer may infer and adopt to a certain extent (Moran, 1996). Thus, increases of prefrontal–posterior EEG coherence during the perception of other’s negative affect may be indicative of the ability to inhibit nascent negative feelings evoked by sympathy with the victim in the joke. This may speed up the perception of amusement elicited by the humor and, consequently, enable faster responses to the amusement ratings. This interpretation is in line with other findings using the same humor paradigm (Papousek, Schulter, Lackner, Samson, & Freudenthaler, 2012; Samson et al., 2012).
Humor susceptibility and positive emotional responsiveness were more strongly predicted by individual differences in the functional (de-)coupling of prefrontal and posterior cortices in the right than in the left hemisphere. Until now, only a small number of studies have investigated potential hemispheric differences in state-dependent EEG coherence changes in the context of affective processing, with inconsistent findings (Miskovic & Schmidt, 2010; Reiser et al., 2012; Schellberg et al., 1990). The greater importance of the right hemisphere in the present study may be in line with evidence that the right hemisphere is implicated in the modulation of the intensity of emotional arousal, independently from hemispheric differences related to the valence or motivational direction of emotional states (Borod, 1992; Gainotti, 2000; Hagemann, Hewig, Naumann, Seifert, & Bartussek, 2005; Papousek, Schulter, & Lang, 2009).
The evidence on hemispheric differences in the context of humor processing is less conclusive. Both hemispheres seem to be involved in the cognitive processing of humor. Some authors have attached greater importance to the left hemisphere in the detection of incongruity and semantic aspects and greater importance to the right than to the left hemisphere in the incongruency resolution and appreciation of humor (Coulson & Williams, 2005; Marinkovic et al., 2011). Likewise, it has been suggested that brain regions in the left and right hemispheres differ with respect to the “coarseness” of semantic coding. Specifically, the left hemisphere is believed to be primarily engaged in relatively fine semantic coding (e.g., focusing activation on a single interpretation or meaning of a verbal stimulus), whereas the right hemisphere is supposed to be engaged in more coarse semantic coding by weakly and diffusely activating alternative or more distant associations (cf. Jung-Beeman, 2005). In support of this framework, cortices in the right hemisphere have been observed as being critically involved in the processing of novel, nonsalient metaphoric meanings (Mashal, Faust, Hendler, & Jung-Beeman, 2007) and in the context of divergent thinking and creative story generation (Howard-Jones, Blakemore, Samuel, Summers, & Claxton, 2005; Martindale, Hines, Mitchell, & Covello, 1984). In line with these considerations, the observed decoupling of right anterior and posterior brain areas during positive affective information processing may also be indicative of a more broadly oriented attentional focus that may, in turn, promote more “coarse” information processing. An individual’s proneness to making less apparent associations may enhance the perceived funniness of the humor (Ruch, 2001; Suls, 1972; Weiss et al., in press). However, recent functional magnetic resonance imaging studies indicated stronger left- than right-hemisphere involvement in the resolution of incongruity (Samson et al., 2008). It seems more likely, therefore, that the greater importance of right- than left-hemisphere prefrontal–posterior (de-)coupling in the present results is related to the processing of social–emotional information rather than to cognitive processes implicated in the perception of humor (cf. Rapp et al., 2008).
When the potential relevance of the interindividual differences in the functional (de-)coupling of prefrontal and posterior cortices during emotional events is considered, it should be kept in mind that only the coherence changes during stimulation with other people’s expressions of positive affect were related to the individual differences in humor susceptibility and positive emotional responsiveness, but not the coherence changes during stimulation with sadness. On the other hand, the present data, as well as previous findings (Reiser et al., 2012), provided indications that decreased functional coupling during the exposure to negative affect expressions is related to a less efficient control and shutting down of negative emotions. Whereas deficient inhibitory processes and the associated sustained processing of negative emotions are common to negative affective dispositions such as depression (Chida & Hamer, 2008; Goeleven, De Raedt, Baert, & Koster, 2006; Nolen-Hoeksema, 1991; Siegle, Granholm, Ingram, & Matt, 2001), positive emotional responsiveness and the maintenance of positive affective states may promote successful functioning (Catalino & Fredrickson, 2011; Eisner, Johnson, & Carver, 2009; Ong et al., 2006; Rottenberg et al., 2002). Nevertheless, there may also be a downside to a more loose prefrontal–posterior coupling and the associated weak control over positive emotional perceptions and representations. Excessive positive emotional responses to positive stimuli, for instance, may be associated with an enhanced risk for mania (Gruber, Johnson, Oveis, & Keltner, 2008).
A potential limitation of the study is that the generalizability of findings obtained in functional connectivity studies may be hampered by the reference used in the EEG recording, because the choice of the reference can influence the coherence measures (Essl & Rappelsberger, 1998; Qin, Xu, & Yao, 2010). A common standard would be desirable to make findings more comparable across studies, but the question of the optimal reference is not resolved (Nunez, 2010). In this study, the problem should be mitigated, because changes within individuals were in the focus of the analysis (rather than absolute values), which were additionally corrected for baseline levels. Nevertheless, the main conclusions drawn from this study remain to be replicated using similar, as well as different, reference montages.
In conclusion, the present study adds to the evidence that interindividual differences in state-dependent changes of prefrontal–posterior EEG beta coherence during affective processing indicate how much impact affectively relevant information has on the individual. A more loose prefrontal–posterior coupling during the exposure to other people’s positive affect expressions predicted greater humor susceptibility, which was assessed several weeks later. In addition, the findings suggested that the propensity to weak control over incoming negative emotional information may hamper the perception of one’s own amusement during the processing of jokes. Together with previous evidence, the findings also indicate that interindividual differences in state-dependent changes of prefrontal–posterior EEG beta coherence during the perception of particular emotions are differentially related to associated psychopathologically relevant traits.
Notes
Please see Samson and Hegenloh (2010) or Samson et al. (2008) for examples of humorous cartoons and nonhumorous pictures.
Analyses in other frequency bands did not reach the significance level.
References
Aftanas, L. I., Lotova, N. V., Koshkarov, V. I., & Popov, S. A. (1998). Non-linear dynamic coupling between different brain areas during evoked emotions: An EEG investigation. Biological Psychology, 48, 121–138. doi:10.1016/S0301-0511(98)00015-5
Borod, J. C. (1992). Interhemispheric and intrahemispheric control of emotion: A focus on unilateral brain damage. Journal of Consulting and Clinical Psychology, 60, 339–348. doi:10.1037/0022-006X.60.3.339
Catalino, L. I., & Fredrickson, B. L. (2011). A Tuesday in the life of a flourisher: The role of positive emotional reactivity in optimal mental health. Emotion, 11, 938–950. doi:10.1037/a0024889
Chida, Y., & Hamer, M. (2008). Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin, 134, 829–885. doi:10.1037/a0013342
Coulson, S., & Williams, S. (2005). Hemispheric asymmetries and joke comprehension. Neuropsychologia, 43, 128–141. doi:10.1016/j.neuropsychologia.2004.03.015
Davidson, R. J. (2002). Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry, 51, 68–80. doi:10.1016/S0006-3223(01)01328-2
Decety, J., & Moriguchi, Y. (2007). The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. BioPsychoSocial Medicine, 1, 22. doi:10.1186/1751-0759-1-22.doi:10.1186/1751-0759-1-22
Diekhof, E. K., Kipshagen, H. E., Falkai, P., Dechent, P., Baudewig, J., & Gruber, O. (2011). The power of imagination: How anticipatory mental imagery alters perceptual processing of fearful facial expressions. Neuroimage, 54, 1703–1714. doi:10.1016/j.neuroimage.2010.08.034
Eisner, L. R., Johnson, S. L., & Carver, C. S. (2009). Positive affect regulation in anxiety disorder. Journal of Anxiety Disorders, 23, 645–649. doi:10.1016/j.janxdis.2009.02.001
Essl, M., & Rappelsberger, P. (1998). EEG coherence and reference signals: Experimental results and mathematical explanations. Medical and Biological Engineering and Computing, 36, 399–406. doi:10.1007/BF02523206
Fazio, R. H. (1990). A practical guide to the use of response latency in social psychological research. In C. Hendrick & M. S. Clark (Eds.), Research methods in personality and social research (pp. 74–97). Newbury: Sage.
Fries, P. (2005). A mechanism for cognitive dynamics: Neuronal communiction through neuronal coherence. Trends in Cognitive Science, 9, 474–480. doi:10.1016/j.tics.2005.08.011
Gainotti, G. (2000). Neuropsychological theories of emotion. In J. C. Borod (Ed.), The neuropsychology of emotion (pp. 214–236). Oxford: Oxford University Press.
Gianotti, L. R. R., Mohr, C., Pizzagalli, D., Lehmann, D., & Brugger, P. (2001). Associative processing and paranormal belief. Psychiatry and Clinical Neuroscience, 55, 595–603. doi:10.1046/j.1440-1819.2001.00911.x
Goeleven, E., De Raedt, R., Baert, S., & Koster, E. H. W. (2006). Deficient inhibition of emotional information in depression. Journal of Affective Disorders, 93, 149–157. doi:10.1016/j.jad.2006.03.007
Gross, J., Schmitz, F., Schnitzler, I., Kessler, K., Shapiro, K., Hommel, B., & Schnitzler, A. (2004). Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proceedings of the National Academy of Sciences USA, 101, 13050–13055. doi:10.1073/pnas.0404944101
Gruber, J., Johnson, S. L., Oveis, C., & Keltner, D. (2008). Risk for mania and positive emotional responding: Too much of a good thing. Emotion, 8, 23–33. doi:10.1037/1528-3542.8.1.23
Hagemann, D. (2004). Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biological Psychology, 67, 157–182. doi:10.1016/j.biopsycho.2004.03.006
Hagemann, D., Hewig, J., Naumann, E., Seifert, J., & Bartussek, D. (2005). Resting brain asymmetry and affective reactivity. Journal of Individual Differences, 26, 139–154. doi:10.1027/1614-0001.26.3.139
Hautzinger, & Bailer, M. (1993). Allgemeine Depressions Skala [General depression scale]. Beltz: Weinheim.
Higashima, M., Takeda, T., Kikuchi, M., Nagasawa, T., Hirao, N., Oka, T., Nakamura, M., & Koshino, Y. (2007). State-dependent changes in intrahemispheric EEG coherence for patients with acute exacerbation of schizophrenia. Psychiatry Research, 149, 41–47. doi:10.1016/j.psychres.2005.05.020
Howard-Jones, P. A., Blakemore, S.-J., Samuel, E. A., Summers, I. R., & Claxton, G. (2005). Semantic divergence and creative story generation: An fMRI investigation. Cognitive Brain Research, 25, 240–250. doi:10.1016/j.cogbrainres.2005.05.013
Johnstone, T., van Reekum, C. M., Urry, H. L., Kalin, N. H., & Davidson, R. J. (2007). Failure to regulate: Counterproductive recruitement of tow-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience, 27, 8877–8884. doi:10.1523/JNEUROSCI.2063-07.2007
Jung-Beeman, M. (2005). Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences, 9, 512–518. doi:10.1016/j.tics.2005.09.009
Kohn, M., Kellermann, T., Gur, R. C., Schneider, F., & Habel, U. (2011). Gender differences in the neural correlates of humor processing: Implications for different processing modes. Neuropsychologia, 49, 888–897. doi:10.1016/j.neuropsychologia.2011.02.010
Kopell, N., Ermentrout, G. B., Whittington, M. A., & Traub, R. D. (2000). Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences USA, 97, 1867–1872. doi:10.1073/pnas.97.4.1867
Koychev, I., Deakin, J. F. W., Haenschel, C., & El-Deredy, W. (2011). Abnormal neural oscillations in schiztopy during a visual working memory task: Support for a deficient top-down network. Neuropsychologia, 49, 2866–2873. doi:10.1016/j.neuropsychologia.2011.06.012
Lackner, H.K., Weiss, E.M., Schulter, G., Hinghofer-Szalkay, H., Samson, A.C., & Papousek, I. (2012). I got it! Transient cardiovascular response to the perception of humor. Manuscript submitted for publication.
Lawrie, S. M., Buechel, C., Whalley, H. C., Frith, C. D., Friston, K. J., & Johnstone, E. C. (2002). Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biological Psychiatry, 51, 1008–1011. doi:10.1016/S0006-3223(02)01316-1
Linden, W., Earle, L., Gerin, W., & Christenfeld, N. (1997). Physiological stress reactivity and recovery: Conceptual siblings separated at birth? Journal of Psychosomatic Research, 42, 117–135. doi:10.1016/S0022-3999(96)00240-1
Marinkovic, K., Baldwin, S., Courtney, M. G., Witzel, T., Dale, A. M., & Halgren, E. (2011). Right hemipshere has the last laugh: Neural dynamics of joke appreciation. Cognitive, Affective, and Behavioral Neuroscience, 11, 113–130. doi:10.3758/s13415-010-0017-7
Martin, R. A. (2007). The psychology of humor. An integrative approach. Burlington: Elsevier Academic Press.
Martindale, C., Hines, D., Mitchell, L., & Covello, E. (1984). EEG alpha asymmetry and creativity. Personality and Individual Differences, 5, 77–86. doi:10.1016/0191-8869(84)90140-5
Mashal, N., Faust, M., Hendler, T., & Jung-Beeman, M. (2007). An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain and Language, 100, 115–126. doi:10.1016/j.bandl.2005.10.005
Miskovic, V., & Schmidt, L. A. (2010). Cross-regional cortical synchronization during affective image viewing. Brain Research, 1362, 102–111. doi:10.1016/j.brainres.2010.09.102
Mohr, C., Graves, R. E., Gianotti, L. R. R., Pizzagalli, D., & Brugger, P. (2001). Loose but normal: A semantic association study. Journal of Psycholinguistic Research, 30, 475–483. doi:10.1023/A:1010461429079
Moran, C. C. (1996). Short-term mood change, perceived funniness, and the effect of humor stimuli. Behavioral Medicine, 22, 32–38. doi:10.1080/08964289.1996.9933763
Nakamura, M., McCarley, R. W., Kubicki, M., Dickey, C. C., Niznikiewicz, M. A., & Shenton, M. E. (2005). Fronto-temporal disconnectivity in schizotypal personality disorder: A diffusion tensor imaging study. Biological Psychiatry, 58, 468–478.
Nolen-Hoeksema, S. (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100, 569–582. doi:10.1037/0021-843X.100.4.569
Nunez, P. (2010). REST: A good idea but not the gold standard. Clinical Neurophysiology, 121, 2177–2180. doi:10.1016/j.clinph.2010.04.029
Ong, A. D., Bergeman, C. S., Bisconti, T. L., & Wallace, K. A. (2006). Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of Personality and Social Psychology, 91, 730–749. doi:10.1037/0022-3514.91.4.730
Papousek, I., & Schulter, G. (1999). Quantitative assessment of five behavioral laterality measures: Distribution of scores and intercorrelations among right-handers. Laterality, 4, 345–362. doi:10.1080/135765099396908
Papousek, I., Schulter, G., & Lang, B. (2009). Effects of emotionally contagious films on changes in hemisphere specific cognitive performance. Emotion, 9, 510–519. doi:10.1037/a0016299
Papousek, I., Freudenthaler, H. H., & Schulter, G. (2011). Typical performance measures of emotion regulation and emotion perception and frontal EEG asymmetry in an emotional contagion paradigm. Personality and Individual Differences, 51, 1018–1022. doi:10.1016/j.paid.2011.08.013
Papousek, I., Reiser, E. M., Weber, B., Freudenthaler, H. H., & Schulter, G. (2012a). Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology, 49, 489–498. doi:10.1111/j.1469-8986.2011.01324.x
Papousek, I., Schulter, G., Lackner, H. K., Samson, A. C., & Freudenthaler, H. H. (2012). Positive emotional responsiveness in the context of humour is related to individual differences in emotion perception and regulation in everyday life. Manuscript submitted for publication.
Phillips, M. L., Ladouceur, C. D., & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 833–857. doi:10.1038/mp.2008.65
Qin, Y., Xu, P., & Yao, D. (2010). A comparative study of different references for EEG default mode network: the use of the infinity reference. Clinical Neurophysiology, 121, 1981–1991. doi:dx.doi.org/10.1016/j.clinph.2010.03.056
Rapp, A. M., Wild, B., Erb, M., Rodden, F. A., Ruch, W., & Grodd, W. (2008). Trait cheerfulness modulates BOLD response in lateral cortical but not limbic brain areas - a pilot fMRI study. Neuroscience Letters, 445, 242–245. doi:10.1016/j.neulet.2008.09.017
Reiser, E. M., Schulter, G., Weiss, E. M., Fink, A., Rominger, C., & Papousek, I. (2012). Decrease of prefrontal-posterior EEG coherence: Loose control during social-emotional stimulation. Brain and Cognition, 80, 144–154. doi:10.1016/j.bandc.2012.06.001
Rominger, C., Weiss, E. M., Fink, A., Schulter, G., & Papousek, I. (2011). Allusive thinking (cognitive looseness) and the propensity to perceive “meaningful” coincidences. Personality and Individual Differences, 51, 1002–1006. doi:10.1016/j.paid.2011.08.012
Rottenberg, J., Kasch, K. L., Gross, J. J., & Gotlib, I. H. (2002). Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion, 2, 135–146. doi:10.1037/1528-3542.2.2.135
Ruch, W., Köhler, G., & van Thriel, C. (1996). Assessing the “humorous temperament”: Construction of the facet and standard trait forms of the State-Trait-Cheerfulness Inventory- STCI. Humor: International Journal of Humor Research, 9, 303–339. doi:10.1515/humr.1996.9.3-4.303
Ruch, W. (2001). The perception of humor. In A. W. Kaszniak (Ed.), Emotion, qualia, and consciousness (pp. 410–425). Tokyo: Word Scientific Publisher.
Ruch, W., & Köhler, G. (2007). A temperament approach to humor. In W. Ruch (Ed.), The sense of humor: Explorations of a personality characteristic (pp. 203–230). Berlin: Mouton de Gruyter.
Samson, A. C., Zysset, S., & Huber, O. (2008). Cognitive humor processing: Different logical mechanisms in non-verbal cartoons – an fMRI study. Social Neuroscience, 3, 125–140. doi:10.1080/17470910701745858
Samson, A. C., & Hegenloh, M. (2010). Structural stimulus properties affect humor processing in individuals with Asperger syndrome. Journal of Autism and Developmental Disorders, 40, 438–447. doi:10.1007/s10803-009-0885-2
Samson, A. C. (2012). The influence of empathizing and systemizing on humor processing: Theory of mind and humor. Humor: International Journal of Humor Research, 25, 75–98. doi:10.1515/humor-2012-0005
Samson, A. C., Lackner, H. K., Weiss, E. M., & Papousek, I. (2012). Perception of other people’s mental states affects humor in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 43, 625–631. doi:10.1016/j.jbtep.2011.08.007
Schellberg, D., Besthorn, C., Klos, T., & Gasser, T. (1990). EEG power and coherence while male adults watch emotional video films. International Journal of Psychophysiology, 9, 279–291. doi:10.1016/0167-8760(90)90060-Q
Schnitzler, A., & Gross, J. (2005). Normal and pathological oscillatory communication in the brain. Nature Review Neuroscience, 6, 285–296. doi:10.1038/nrn1650
Sepulcre, J., Liu, H., Talukdar, T., Martincorena, I., Yeo, B. T., & Buckner, R. L. (2010). The organization of local and distant functional connectivity in the human brain. Plos Computational Biology, 6, e1000808. doi:10.1371/journal.pcbi.1000808
Shammi, P., & Stuss, D. T. (2003). The effects of normal aging on humor. Journal of the International Neuropsychological Society, 9, 855–863. doi:10.1017/S135561770396005X
Siegle, G. J., Granholm, E., Ingram, R. E., & Matt, G. E. (2001). Pupillary and reaction rime measures of sustained processing of negative information in depression. Biological Psychiatry, 49, 625–636. doi:10.1016/S0006-3223(00)01024-6
Srinivasan, R., Winter, W. R., Ding, J., & Nunez, P. L. (2007). EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. Journal of Neuroscience Methods, 166, 41–52. doi:10.1016/j.jneumeth.2007.06.026
Steingrüber, H., & Lienert, G. (1971). Hand-Dominanz-Test. Göttingen: Hogrefe.
Steketee, G. S., & Chambless, D. L. (1992). Methodological issues in the prediction of treatment outcome. Clinical Psychology Review, 12, 387–400. doi:10.1016/0272-7358(92)90123-P
Suls, J. M. (1972). A two-stage model for the appreciation of jokes and cartoons: An information processing analysis. In J. H. Goldstein & P. E. McGhee (Eds.), The psychology of humor (pp. 81–100). New York: Academic Press.
Vercammen, A., Knegtering, H., den Boer, J. A., Liemburg, E. J., & Aleman, A. (2010). Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biological Psychiatry, 67, 912–918. doi:10.1016/j.biopsych.2009.11.017
Vuilleumier, P., & Driver, J. (2007). Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society B, 362, 873–855. doi:10.1098/rstb.2007.2092
Weber, B., Papousek, I., & Schulter, G. (2011, July). Acoustical mood induction - development of new stimulus material (ECOS - Emotionally Contagious Sound Clips). Poster, presented at the 12th European Congress of Psychology, Istanbul, Turkey.
Weiss, E. M., Gschaidbauer, B. C., Samson, A. C., Steinbäcker, K., Fink, A., & Papousek, I. (in press). From Ice age to Madagascar. Appreciation of slapstick humor in children with Asperger’s syndrome. Humor: International Journal of Humor Research, in press.
Winterer, G., Coppola, R., Egan, M. F., Goldberg, T. E., & Weinberger, D. R. (2003). Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biological Psychiatry, 54, 1181–1192. doi:10.1016/S0006-3223(03)00532-8
Wood, A. M., Taylor, P. J., & Joseph, S. (2010). Does the CES-D measure a continuum from depression to happiness? Comparing substantive and artifactual models. Psychiatry Research, 177, 120–123. doi:10.1016/j.psychres.2010.02.003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papousek, I., Reiser, E.M., Weiss, E.M. et al. State-dependent changes of prefrontal–posterior coupling in the context of affective processing: Susceptibility to humor. Cogn Affect Behav Neurosci 13, 252–261 (2013). https://doi.org/10.3758/s13415-012-0135-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-012-0135-5