Abstract
Typically, response-repetition effects are obtained in task-switching experiments: In task repetitions, performance is enhanced when the response, too, repeats (response-repetition benefits), whereas in task switches, performance is impaired when the response repeats (response-repetition costs). A previous study introduced cue modality switches in a cued task-switching paradigm with visual stimuli and obtained enhanced response-repetition benefits when the cue modality repeated (Koch, Frings, & Schuch Psychological Research, 82, 570–579, 2018). In the present study, we aimed to replicate this finding with auditory stimuli (Exp. 1), and further examined whether response-repetition effects could be modulated by introducing stimulus modality switches (Exp. 2). We found clear evidence that the cue modality and stimulus modality affect task switch costs. The task switch costs were higher with a repeated cue modality or stimulus modality. However, cue modality switches or stimulus modality switches did not affect the response-repetition effects. We suggest that response-repetition effects are elicited by response-associated bindings, which are not necessarily affected by all episodic task features to the same extent. Our results are also in line with theoretical accounts that assume a hierarchical organization of task selection and response selection.
Similar content being viewed by others
Variations of human performance in changing contexts have often been studied using the task-switching paradigm. In cued task switching, a cue is presented prior to each stimulus, indicating which task must be executed. For example, participants are asked to categorize a number stimulus as odd versus even (parity task) or as smaller versus larger than 5 (magnitude task). They respond by pressing one of two keys (left vs. right key), thereby indicating an “odd” versus “even” or a “smaller” versus “larger” response. The same task (task repetition) or a different task (task switch) can be required in subsequent trials, which typically results in task switch costs—longer reaction time (RT) and more errors in task switches than in task repetitions (Jersild, 1927; for reviews, see Jost, De Baene, Koch, & Brass, 2013; Kiesel et al., 2010; Koch, Poljac, Müller, & Kiesel, 2018; Monsell, 2003; Vandierendonck, Liefooghe, & Verbruggen, 2010).
A robust finding in task-switching studies is the pattern of response-repetition effects (RR effects). Various studies have shown that in task repetitions, response-repetition benefits (RR benefits) are obtained: Response repetitions are faster and more accurate than response switches. In task switches, response-repetition costs (RR costs) occur: Response repetitions are slower and less accurate than response switches (e.g., Altmann, 2011; Druey, 2014; Hübner & Druey, 2006; Kleinsorge, 1999; Koch, Frings, & Schuch, 2018; Koch, Schuch, Vu, & Proctor, 2011; Meiran, 2000; Quinlan, 1999; Rogers & Monsell, 1995; Schuch & Koch, 2004, 2010; Seibold et al., 2019; for a review, see Gade, Schuch, Druey, & Koch, 2014).
Different theoretical accounts have proposed explanations for RR effects (for an overview, see Druey, 2014; Hübner & Druey, 2006). In the following paragraphs, the episodic retrieval account (e.g., Altmann, 2011), the priming and inhibition account (e.g., Hübner & Druey, 2006), and the hybrid, dual-mechanism account (Koch, Frings, & Schuch, 2018) will be described.
According to the episodic retrieval account (e.g., Altmann, 2011; for a similar idea, see Schuch & Koch, 2004) all episodic task features (termed “event files” in the theory of event coding by Hommel, Müsseler, Aschersleben, & Prinz, 2001) are bound when a task is executed. This binding of episodic task features includes, for example, the cue, the stimulus category, the stimulus modality, and the response category. For instance, when the stimulus category “odd numbers” in a parity task and a “left” response are bound, subsequent performance is enhanced (RR benefits) when this binding can be reused on another “odd number” in a parity task that also requires a “left” response. In contrast, performance is impaired (RR costs) when at least one feature of the current task episode differs from the previous task episode while other features remain the same. For example, consider a response repetition that is newly associated with the stimulus category “smaller than 5” in a magnitude task. The previous episode is reactivated by repeated episodic task features, but this activation creates interference that needs to be overcome to execute a task switch (this process is called “unbinding” in Schuch & Koch, 2004). In the case of a complete switch of all episodic task features, neither positive effects due to the retrieved binding nor negative effects due to interference occur.
Alternatively, according to the priming and inhibition account (Druey, 2014; Druey & Hübner, 2008; Grzyb & Hübner, 2012; Hübner & Druey, 2006; Rogers & Monsell, 1995; Steinhauser, Hübner, & Druey, 2009), responses are automatically inhibited after their execution. It has been suggested that this mechanism prevents the accidental reexecution of the same response in subsequent trials. RR costs originate from this automatic response inhibition that must be overcome when the response repeats. Additionally, this account postulates that stimulus categories elicit a positive priming effect when they are repeated in subsequent trials. RR benefits are explained by this positive stimulus category priming, which outweighs the automatic inhibition that follows the response execution.
According to the hybrid, dual-mechanism account (Koch, Frings, & Schuch, 2018), both mechanisms (episodic binding and response inhibition) play parts in the origin of the typical cost–benefit pattern of response-repetition effects. Koch, Frings, and Schuch (2018) introduced cue modality switches (visual vs. auditory) in order to vary the similarity of the encoding and retrieval context of episodic bindings. By doing this, a contextual facilitation of the retrieval of the episodic binding was successfully established—RR benefits in task repetitions were higher with a repeated than with a switched cue modality. However, RR costs in task switches remained unaffected by cue modality switches, which is in line with a carryover of response inhibition that is independent of the episodic context. The authors concluded that RR benefits originate mainly from episodic bindings, whereas RR costs are more strongly driven by the carryover of response inhibition.
In Experiment 1 of the present study, we examined the effect of cue modality switches (visual vs. auditory) on RR effects using auditory stimuli. In Experiment 2, we introduced additional stimulus modality switches (visual vs. auditory) in order to examine whether the impact on RR effects is similar to the impact of cue modality switches.
Experiment 1
In Experiment 1, we expected to find the typical cost–benefit pattern of RR effects, and further expected higher RR benefits in cue modality repetitions than in cue modality switches, due to the higher contextual overlap of the episodic bindings (see also Koch, Frings, & Schuch, 2018). However, cue modality switches should not modulate RR costs in task switches, since the latter are assumed to be due to a carryover of response inhibition, which should arise independently of episodic bindings.
Method
Participants
Twenty-four students (17 female, four left-handed), between 19 and 33 years of age (M = 21.71 years, SD = 3.31), from RWTH Aachen University took part in the experiment. All participants had normal or corrected-to-normal vision and reported no hearing problems. The sample size was based on Koch, Frings, and Schuch (2018) and Seibold et al. (2019), who obtained reliable response-repetition effects with this sample size.
Apparatus, stimuli, and tasks
The experiment was implemented with PsychoPy 2.0 (Peirce, 2009) and run on a computer with the Linux operating system. The stimuli were the numbers 1–9, excluding 5, which were presented auditorily by a male speaker via headphones (Trust 16904 Quasar Headset). The auditory stimuli were recorded at the Institute of Technical Acoustics of RWTH Aachen University in an anechoic chamber and were adjusted for subjective loudness (DIN 45631) as well as for equal duration (600 ms). The participants were seated at about a 60-cm viewing distance from the monitor.
The participants had to execute either a magnitude task (smaller vs. larger than 5) or a parity task (odd vs. even) on the stimuli. Responses were made on a German computer keyboard (QWERTZ) by pressing the left (“V”) or right (“B”) response keys (labeled with red stickers) with the left or right index finger, respectively. While the stimulus–response mapping for the magnitude task was held compatible with the mental number line, which maps smaller numbers to the left and greater numbers to the right (Dehaene, Bossini, & Giraux, 1993), the stimulus–response mapping for the parity task was counterbalanced across participants. At the beginning of each trial, a visual or auditory cue indicated which task had to be executed. Cues similar to those in Koch, Frings, and Schuch (2018) were used, to ensure the comparability of our results to those from the preceding study.Footnote 1 On half of the trials, a visual cue composed of a black bar (average height 1.3 cm, average width 4.4 cm) was presented in either the upper or the lower part of the computer screen (5.2 cm above vs. below the center of the screen). On the other half of the trials, an auditory cue that was either low or high in pitch (262 vs. 748 Hz) was presented via headphones. The duration of the visual cues was 200 ms, whereas the auditory cues lasted for 150 ms. The auditory cues were presented a slightly shorter time to avoid an overlap of the auditory cues and the stimuli. To ensure comparable conditions for both cue modalities, the cue–stimulus interval (CSI) lasted 200 ms for both cue modalities. The cue–task mapping was counterbalanced across participants, and the assignment of “low” versus “high” cues remained constant across both modalities.
Procedure
The procedure started with a questionnaire asking for the participants’ informed consent and for biographical data including age, handedness, gender, and hearing/vision problems. This was followed by performance of the experiment, which concluded with a questionnaire asking for applied strategies and comments. One experimental session took about 30 min. The experiment included a sequence of training trials (32 trials) and five experimental blocks (120 trials in each block), resulting in 632 trials in total.
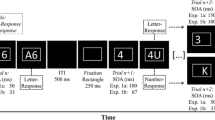
Each trial started with the presentation of a visual or auditory cue, which was followed by a fixed CSI of 200 ms from the cue onset. After this, an auditory stimulus was presented. After the response was executed, a fixed response–cue interval (RCI) of 1,400 ms followed that initiated the next trial. In the case of an incorrect response, visual feedback was given to participants in the form of the word “Fehler” (German for “error”), which was printed in red font color on the screen immediately after the response for 500 ms, and the intertrial interval was prolonged accordingly. An overview of the time course of two experimental trials can be found in Fig. 1.
Time course of two experimental trials and definition of temporal intervals. A visual or auditory cue indicating the relevant task was followed by the onset of the number stimulus, which was presented either entirely auditorily (Exp. 1) or visually versus auditorily (Exp. 2). Responses were made by pressing the left (“V”) or right (“B”) response keys. The response–stimulus interval (RSI) and the cue–stimulus interval (CSI) were fixed.
Each cue and each stimulus appeared equally often, and the numbers of switches and repetitions were equal for each feature (task, correct response, and cue modality). Furthermore, the same number of stimulus pairs required the same response (congruent stimuli) as required opposite responses (incongruent stimuli) for both tasks. As in the study by Seibold et al. (2019), all immediate stimulus repetitions (repetition of the same stimulus from trial n–1 to trial n) and direct stimulus-task repetition episodes (repetition of the same stimulus that had occurred during the last episode of the same task) were excluded from the data analysis (from here onward, termed “stimulus repetitions”). This was done in order to measure RR effects without any confounding from stimulus repetition effects. However, these stimulus repetitions were not excluded from occurring in the experiment, because this would cause a local switch bias for the stimulus categories, which could distort the response-repetition effects (see Altmann, 2011).Footnote 2
Design
The independent within-subjects variables were task transition (task repetition vs. task switch), response transition (response repetition vs. response switch), and cue modality transition (cue modality repetition vs. cue modality switch). The dependent variables were reaction time (RT) and error rates. Significance was tested at α = .05.
Terminology
Please note that task transition effects and modality transition effects are termed “task switch costs” and “modality switch costs” in the present article, reflecting the presumed disruptive effect on performance of the required attentional shift between tasks and modalities. These effects could equally be described using the terms “task repetition benefits” and “modality repetition benefits,” focusing on the presumed reapplication of the previously formed episodic binding enhancing performance. To be clear and coherent, we use the term “switch costs” throughout the article.
Results and discussion
The first trial of each block, the practice trials, and the aforementioned stimulus repetitions (16.6%) were excluded from the data analyses. Furthermore, trials following an error (5.7%) were excluded, because these trials cannot be classified as switch or repetition trials. For the RT analysis, all erroneous trials (5.7%) were discarded, and to identify outliers, all RTs were z-transformed separately for each participant, and trials with z > 3 or z < – 3 (1.4%) were not included in the RT analysis.Footnote 3 The data of one participant had to be excluded from the analyses entirely, because an incorrect response was given on 26.2% of the trials. An additional participant was tested as a substitute. The RT and error rates were analyzed in separate 2 × 2 × 2 analyses of variance (ANOVAs) with repeated measures.
RT analysis
The ANOVA on mean RTs yielded a main effect of task transition, F(1, 23) = 48.78, p < .001, ηp2 = .68, indicating task switch costs of 117 ms, with higher RTs for task switches than for task repetitions. The main effect of cue modality transition was significant, too, F(1, 23) = 30.87, p < .001, ηp2 = .57, indicating cue modality switch costs of 97 ms, with higher RTs in trials with a switched than with a repeated cue modality.
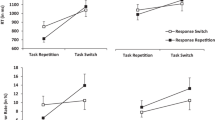
Here and in the following experiment, both RR benefits and costs are computed as response switches minus response repetitions. As expected, task transition and response transition entered into a significant interaction, F(1, 23) = 4.82, p < .05, ηp2 = .17, indicating RR benefits of 49 ms for task repetitions and RR costs of – 28 ms for task switches. Hence, the typical response-repetition interaction pattern was successfully replicated in the present study employing auditory stimuli. However, the three-way interaction of task transition, response transition, and cue modality transition was not significant, F(1, 23) = 0.52, p = .48, ηp2 = .02 (see Fig. 2).
Experiment 1: Mean reaction times (RTs; upper panel, in milliseconds) and mean error rates (lower panel, as percentages) as a function of task transition, response transition, and cue modality transition. The patterns of response-repetition effects are comparable for cue modality repetitions and cue modality switches. The error bars represent the standard errors of the means.
Additionally, task transition interacted with cue modality transition, F(1, 23) = 19.48, p < .001, ηp2 = .46, indicating higher task switch costs in trials with a repeated cue modality (182 ms) than with a switched cue modality (54 ms). All other effects were nonsignificant, Fs < 0.61, ps > .44.
Error analysis
The corresponding ANOVA on error rates also yielded a main effect of task transition, F(1, 23) = 4.62, p < .05, ηp2 = .17, indicating task switch costs of 1.3%, with more errors for task switches than for task repetitions. The main effect of cue modality transition was not significant, but it showed a nonsignificant trend in the same direction as for the RTs, F(1, 23) = 1.34, p = .26, ηp2 = .06, with numerically more errors in trials with a switched cue modality (5.7%) than with a repeated cue modality (5.1%).
Again, task transition and response transition entered into a significant interaction, F(1, 23) = 7.69, p < .05, ηp2 = .25, indicating numerically negative RR benefits of – 0.2% in task repetitions, and large RR costs of – 3.3% in task switches. An additional main effect of response transition was significant, F(1, 23) = 9.55, p < .01, ηp2 = .29, with generally more errors in response repetitions (6.2%) than in response switches (4.5%). Corresponding to the RT analysis, the expected three-way interaction of task transition, response transition, and cue modality transition was not significant, F(1, 23) = 0.68, p = .42, ηp2 = .03 (see Fig. 2). All other effects were nonsignificant, Fs < 0.23, ps > .64.
In sum, the typical interaction pattern of task transition and response transition, indicating RR benefits in task repetitions and RR costs in task switches, was replicated. However, we did not find evidence that cue modality repetitions result in increased RR benefits as compared to cue modality switches. Hence, with auditory stimuli, we did not replicate the modulation of the pattern of RR effects by cue modality switches that was reported by Koch, Frings, and Schuch (2018), who used visual stimuli.
However, it is notable that in the RTs, task transition and cue modality transition entered into a significant interaction: Task switch costs were higher for cue modality repetitions than for cue modality switches (i.e., 182 vs. 54 ms). In this matter, we replicated the binding effects of the cue modality with the task reported by Koch, Frings, and Schuch (2018).
Experiment 2
In Experiment 2, we additionally varied the stimulus modality in order to further examine whether RR effects are modulated by cue modality and stimulus modality switches, and we expected to find a modulation of RR effects by cue modality switches and stimulus modality switches: In task repetitions, cue modality repetitions and stimulus modality repetitions should result in higher RR benefits than do cue modality switches and stimulus modality switches. At the same time, in task switches, cue modality switches and stimulus modality switches should not modulate RR costs.
Furthermore, the impact of stimulus modality switches on RR effects may be more pronounced than the impact of cue modality switches, because the stimulus modality may be bound more directly to the response in the episode than is the cue modality. To be precise, the stimulus modality may be more directly bound to the response-associated stimulus category, because it relates to the same “object” (the stimulus), as compared to the cue modality, which is only indirectly related to response selection (via cue-based task selection).
Method
Participants
Twenty-four new participants (18 female, three left-handed), between 18 and 28 years of age (M = 21.58 years, SD = 3.19), from RWTH Aachen University took part in the experiment. All participants had normal or corrected-to-normal vision and reported no hearing problems.
Apparatus, stimuli, tasks, and procedure
The apparatus, cues, tasks, and procedure were the same as in Experiment 1. However, in Experiment 2 both auditory and visual stimuli were used. The stimuli were the numbers 1–9, excluding 5, and were presented either auditorily by a male speaker via the same headphones as in Experiment 1, or visually on a computer monitor (22-in. LG 22MB65PM). The auditory stimuli were the same as in the preceding experiment. The visual stimuli (average height 6.3 cm, average width 4.0 cm, Arial font) were presented centrally in black font color on a white background. To ensure comparable conditions for the different presentation modalities, all visual stimuli disappeared after 600 ms, corresponding to the duration of the auditory stimuli (see Fig. 1).
Design
The independent within-subjects variables were task transition (task repetition vs. task switch), response transition (response repetition vs. response switch), stimulus modality transition (stimulus modality repetition vs. stimulus modality switch), and cue modality transition (cue modality repetition vs. cue modality switch). RTs and error rates remained as the dependent variables.
Results and discussion
As in the first experiment, the first trial of each block, the practice trials, the stimulus repetitions (16.5%), and trials following an error (7.8%) were excluded from the data analyses. Furthermore, erroneous trials (7.8%) and outliers (1.5%) were not included in the RT analysis. The RTs and error rates were analyzed in separate 2 × 2 × 2 × 2 ANOVAs with repeated measures.
RT analysis
The ANOVA of mean RTs yielded a main effect of task transition, F(1, 23) = 15.93, p < .01, ηp2 = .42, indicating task switch costs of 149 ms, with higher RTs for task switches than for task repetitions. The main effect of cue modality transition was significant, too, F(1, 23) = 16.61, p < .01, ηp2 = .43, indicating cue modality switch costs of 147 ms, with higher RTs for cue modality switches than for cue modality repetitions. The main effect of stimulus modality transition was not significant, F(1, 23) = 3.49, p = .08, ηp2 = .14, but suggested a numerical pattern, with higher RTs for stimulus modality switches (1,410 ms) than for stimulus modality repetitions (1,369 ms).
As expected, task transition and response transition entered into a significant interaction, F(1, 23) = 14.42, p < .01, ηp2 = .40, indicating RR benefits of 76 ms for task repetitions and RR costs of – 75 ms for task switches. Hence, the typical pattern of RR effects was again successfully replicated. Importantly, the expected three-way interaction of task transition, response transition, and cue modality transition was not significant, F(1, 23) = 0.86, p = .37, ηp2 = .04 (see Fig. 3). Likewise, the expected three-way interaction of task transition, response transition, and stimulus modality transition was also not significant, F(1, 23) = 0.21, p = .65, ηp2 = .01 (see Fig. 3).
Experiment 2: Mean reaction times (RTs; upper panel, in milliseconds) and mean error rates (lower panel, as percentages) as a function of task transition, response transition, cue modality transition, and stimulus modality transition. The patterns of response-repetition effects are comparable for stimulus modality transitions and cue modality transitions (the left four panels depict cue modality repetitions, and the right four panels depict cue modality switches). The error bars represent the standard errors of the means.
However, as in Experiment 1, task transition interacted with cue modality transition, F(1, 23) = 17.03, p < .001, ηp2 = .44, indicating higher task switch costs for cue modality repetitions (224 ms) than for cue modality switches (76 ms). A comparable interaction was also obtained for task transition and stimulus modality transition, F(1, 23) = 4.49, p < .05, ηp2 = .17, indicating higher task switch costs for stimulus modality repetitions (187 ms) than for stimulus modality switches (112 ms). All other effects were nonsignificant, Fs < 1.76, ps > .19.
Error analysis
The corresponding ANOVA of error rates also yielded a main effect of task transition, F(1, 23) = 5.44, p < .05, ηp2 = .19, indicating task switch costs of 2.8%, with more errors for task switches than for task repetitions. Neither the main effect of cue modality transition, F(1, 23) = 2.20, p = .15, ηp2 = .09, nor the main effect of stimulus modality transition, F(1, 23) = 0.80, p = .38, ηp2 = .03, was significant.
Again, task transition and response transition entered into a significant interaction, F(1, 23) = 16.19, p < .01, ηp2 = .41, indicating small RR benefits of 0.5% for task repetitions and RR costs of – 5.7% for task switches. Moreover, the main effect of response transition was significant, F(1, 23) = 6.94, p < .05, ηp2 = .23, with generally more errors for response repetitions (8.6%) than for response switches (6.0%). Corresponding to the RT analysis, the expected three-way interaction of task transition, response transition, and cue modality transition was not significant, F(1, 23) = 1.56, p = .22, ηp2 = .06 (see Fig. 3). Likewise, the expected three-way interaction of task transition, response transition, and stimulus modality transition was also not significant, F(1, 23) = 1.42, p = .25, ηp2 = .06 (see Fig. 3). All other effects were nonsignificant, Fs < 1.93, ps > .17.
In Experiment 2, the typical pattern of RR effects was replicated, but no evidence for a modulation of the RR effects by cue modality switches or by the newly introduced stimulus modality switches could be found. Yet, as in Experiment 1, we found that task switch costs were higher when either the cue modality or the stimulus modality repeated, confirming the findings reported by Koch, Frings, and Schuch (2018) regarding the binding of the cue modality to the task.
Stimulus modality-specific analyses
Further analyses on subsets of the dataset of Experiment 2 were conducted to rule out a systematic influence of the auditory versus visual stimulus modality on the relevant three-way interaction of the cue modality transition with RR effects. The reported analyses included only trials in a specific stimulus modality—auditory versus visual stimulus modality trials—and were constrained to trials in which the same stimulus modality was presented sequentially (i.e., stimulus modality repetition trials). Consequently, these modality-specific post-hoc analyses were conducted on a rather small number of trials per condition. In the case that a participant did not have at least one data point for each condition, that participant’s data were excluded from the analysis.
RT analysis for auditory trials
The ANOVA of mean RTs for auditory stimulus modality repetitions yielded a main effect of task transition, F(1, 19) = 10.67, p < .01, ηp2 = .36, indicating task switch costs of 198 ms, with higher RTs for task switches than for task repetitions. The main effect of cue modality transition was significant, too, F(1, 19) = 13.32, < .01, ηp2 = .41, indicating cue modality switch costs of 196 ms, with higher RTs for cue modality switches than for cue modality repetitions. Task transition and response transition showed a marginally significant interaction, F(1, 19) = 3.86, p = .06, ηp2 = .17, indicating numerical RR benefits of 32 ms for task repetitions and RR costs of – 122 ms for task switches.
Again, task transition interacted with cue modality transition, F(1, 19) = 11.91, p < .01, ηp2 = .39, indicating higher task switch costs for cue modality repetitions (293 ms) than for cue modality switches (102 ms). Neither the main effect of response transition, F(1, 19) = 1.27, p = .27, ηp2 = .06, nor the expected three-way interaction of task transition, response transition, and cue modality transition was significant, F(1, 19) = 1.43, p = .25, ηp2 = .07.
Error analysis for auditory trials
The corresponding ANOVA of error rates for auditory stimulus modality repetitions yielded no main effect of task transition, F(1, 19) = 2.95, p = .10, ηp2 = .13, but there was a main effect of cue modality transition, F(1, 19) = 9.16, p < .01, ηp2 = .33, indicating cue modality switch costs of 3.2%, with more errors on cue modality switches than on cue modality repetitions. Task transition and response transition entered into a significant interaction, F(1, 19) = 5.11, p < .05, ηp2 = .21, indicating RR benefits of 0.6% for task repetitions and RR costs of – 5.3% for task switches. All other effects were nonsignificant, Fs < 0.97, ps > .34.
RT analysis for visual trials
The ANOVA of mean RTs for visual stimulus modality repetitions yielded a main effect of task transition, F(1, 16) = 13.79, p < .01, ηp2 = .46, indicating task switch costs of 238 ms, with higher RTs for task switches than for task repetitions. The main effect of cue modality transition was significant, too, F(1, 16) = 11.86, p < .01, ηp2 = .43, indicating cue modality switch costs of 170 ms, with higher RTs for cue modality switches than for cue modality repetitions. Task transition and response transition entered into a significant interaction, F(1, 16) = 5.06, p < .05, ηp2 = .24, indicating RR benefits of 167 ms for task repetitions and RR costs of – 51 ms for task switches. Task transition also interacted with cue modality transition, F(1, 16) = 7.62, p < .05, ηp2 = .32, indicating higher task switch costs for cue modality repetitions (325 ms) than for cue modality switches (149 ms). Neither the main effect of response transition, F(1, 16) = 1.57, p = .23, ηp2 = .09, nor the expected three-way interaction of task transition, response transition, and cue modality transition was significant, F(1, 16) = 0.04, p = .86, ηp2 = .002.
Error analysis for visual trials
The corresponding ANOVA of error rates for visual stimulus modality repetitions yielded neither a main effect of task transition, F(1, 21) = 1.71, p = .21, ηp2 = .08, nor a main effect of cue modality transition, F(1, 21) = 0.09, p = .77, ηp2 = .004, nor a main effect of response transition, F(1, 21) = 2.11, p = .16, ηp2 = .09. However, task transition and response transition entered into a significant interaction, F(1, 21) = 9.02, p < .01, ηp2 = .30, indicating RR benefits of 1.8% for task repetitions and RR costs of – 7.1% for task switches. Again, the relevant three-way interaction of task transition, response transition, and cue modality transition did not reach significance, F(1, 21) = 2.74, p = .11, ηp2 = .12. All other effects were also nonsignificant, Fs < 0.28, ps > .60. The results indicate that the data patterns are similar when analyzing stimulus modality repetitions for the auditory or the visual stimulus modality separately.
Combined analysis of Experiments 1 and 2
A between-experiment comparison was conducted, to confirm the finding of a nonsignificant interaction of cue modality transition with RR effects in our two experiments. Specifically, the present analysis was conducted to rule out the possibility that our nonsignificant results were due to insufficient power. An analysis is reported in which experiment (Exp. 1 vs. Exp. 2) was introduced as an additional between-subjects variable, in addition to the within-subjects variables task transition, response transition, and cue modality transition. The within-subjects variable stimulus modality transition was omitted from the combined analysis, as it was only introduced in Experiment 2 (i.e., the data were averaged over stimulus modality repetitions and switches). For RTs, task transition and response transition interacted significantly, F(1, 46) = 22.16, p < .001, ηp2 = .33, indicating RR benefits of 62 ms for task repetitions and RR costs of – 49 ms for task switches. The three-way interaction of task transition, response transition, and cue modality transition remained nonsignificant, F(1, 46) = 1.64, p = .21, ηp2 = .03, and was also not modulated by the experiment variable, F(1, 46) = 0.22, p = .64, ηp2 < .01. Neither the main effect of the experiment variable nor any other interaction with that variable was significant, Fs < 2.96, ps > .09.
In the error rates, again, task transition and response transition interacted significantly, F(1, 46) = 25.38, p < .001, ηp2 = .36, indicating RR benefits of 0.3% for task repetitions and RR costs of – 4.2% for task switches. Corresponding to the RT data, neither the three-way interaction of task transition, response transition, and cue modality transition, F(1, 46) = 2.14, p = .15, ηp2 = .04, nor the main effect nor any interaction with the experiment variable was significant, Fs < 2.67, ps > .14. Hence, the combined analysis of Experiments 1 and 2 did not reveal any evidence for a modulation of RR effects by cue modality switches.
To further support the present results, we applied Bayes statistics, quantifying the probability ratio of obtaining the pooled data across Experiments 1 and 2, given that the null hypothesis (H0, no difference) was true versus given that the alternative hypothesis (H1, difference) was true. We used the method suggested by Rouder, Speckman, Sun, Morey, and Inverson (2009), of computing one-sample t tests and the Bayes factor (BF) with a scale of r = 1, assuming that the effect size was normally distributed. Regarding the difference in RR benefits in task repetitions for cue modality repetitions versus switches, the Bayes factor revealed moderate evidence in favor of the null hypothesis in the RT data, t(47) = 0.86, p = .39, BF01 = 6.156. Regarding the difference in RR benefits in task repetition error rates for cue modality repetitions versus switches, t(47) = 1.95, p = .06, BF01 = 1.478, the Bayes factor neither supported nor ruled out the null nor the alternative hypothesis, allowing no strong conclusion.
Regarding the difference in RR benefits in task repetitions for stimulus modality repetitions versus switches in Experiment 2, the Bayes factors again revealed moderate evidence in favor of the null hypothesis, in both the RT data, t(23) = 0.81, p = .43, BF01 = 4.670, and the error rates, t(23) = 0.08, p = .94. BF01 = 6.358.
General discussion
The present study joins the ranks of multiple studies that have examined response-repetition effects in task switching (e.g., Altmann, 2011; Druey, 2014; Hübner & Druey, 2006; Kleinsorge, 1999; Koch, Frings, & Schuch, 2018; Koch et al., 2011; Meiran, 2000; Quinlan, 1999; Rogers & Monsell, 1995; Schuch & Koch, 2004, 2010; Seibold et al., 2019; for a review, see Gade et al., 2014). RR effects were consistently found in the RTs and the error rates of our two experiments, with RR benefits for task repetitions and RR costs for task switches.
Episodic binding of the cue modality and the stimulus modality to the task
In the RTs, both cue modality switches (Exps. 1 and 2) and stimulus modality switches (Exp. 2) interacted significantly with task switches: Task switch costs were higher for cue modality repetitions and stimulus modality repetitions than when the cue modality or stimulus modality switched. This effect can be interpreted either as a disruptive effect on performance of modality switches and task switches (i.e., switch costs) or as an enhancing effect (i.e., repetition benefits) caused by modality repetitions and task repetitions. Please note that both terms, “switch costs” and “repetition benefits,” describe the difference between performance in repetitions and switches, but they refer to different baselines.
The potential disruption of task selection may be due to the required attentional shift between the different tasks or modalities (see the reports of stimulus modality switch costs in, e.g., Lukas, Philipp, & Koch, 2014; Quinlan & Hill, 1999; Spence & Driver, 1997). Task switch costs can also be interpreted in the light of episodic binding: When the task and the cue modality or stimulus modality repeat, the previously formed episodic binding (i.e., of the task and the cue modality or of the task and the stimulus modality) can be reapplied, thus enhancing performance. In the case of a switch of either the task, the cue modality or the stimulus modality, the previous task–modality binding must be overcome.
The interactions of task switches and cue modality switches and of task switches and stimulus modality switches provides evidence for episodic binding, offering a viable explanation for the higher task switch costs in cue modality repetitions and stimulus modality repetitions. Notably, our results are in line with the data pattern reported by Koch, Frings, and Schuch (2018) regarding the binding of the task and the cue modality. However, the specific influence of cue modality switches on RR benefits that was observed by Koch, Frings, and Schuch. in two experiments using visual stimuli (and replicated in corresponding conditions by Seibold et al., 2019), could not be found under the present conditions.
Response-associated binding
Contrary to expectations based on the hybrid, dual-mechanism account of RR effects (Koch, Frings, & Schuch, 2018), cue modality switches and stimulus modality switches did not significantly modulate the RR effects in our experiments using auditory stimuli. Our findings suggest that, in our experiments, the cue modalities and the stimulus modalities were not integrated into episodic bindings together with the responses. Hence, response selection was unaffected by cue or stimulus modality switches, suggesting that responses were selected on the basis of abstract cue or stimulus categories, uninfluenced by the response-irrelevant perceptual properties of the cues or stimuli. One possible explanation for the absent effect of cue or stimulus modality switches on response selection may be that the response-associated binding of episodic task features does not include all episodic task features, but mainly response-relevant features. In the present experimental setup employing auditory stimuli, the response-associated binding seems to have included abstract cue and stimulus categories rather than response-irrelevant specific perceptual properties of the cues or stimuli.
However, interpretations regarding the independence of the established bindings from response-irrelevant perceptual properties of the task episode should be reviewed carefully, since various studies have shown evidence for the integration of distractors into episodic bindings (for evidence of distractor–response binding, see, e.g., Frings, 2011; Frings, Rothermund, & Wentura, 2007): Employing a prime–probe design, Frings et al. (2007) found evidence that performance in response repetitions is enhanced when a response-irrelevant distractor stimulus also repeats. In contrast, performance in response repetitions is impaired when a response-irrelevant distractor switches. Frings and colleagues argued that the retrieval of episodic bindings can be triggered by response-irrelevant distractors (for a review of distractor processing, see Frings, Schneider, & Moeller, 2014). However, distractor features were only included when they matched the task-relevant dimension of the target stimulus (e.g., the distractor’s location feature was included in the episodic binding when the attended target stimulus’ relevant dimension was also its location). It remains to be determined which differences between the cued task-switching paradigm of the present study and the prime–probe design specifically explain the different theoretical conclusions regarding the binding of response-irrelevant episodic task features.
Rather than assuming that some response-irrelevant episodic features are not included in the binding at all, the notion of intentional weighting (see Memelink & Hommel, 2013) assumes that different episodic task features are included in the binding with different weights. Hence, in our experimental design, the task-irrelevant episodic features of the cue modality and stimulus modality might be bound to the response only with a nonsignificant attentional weight.
When comparing the present study with the study by Koch, Frings, and Schuch (2018), one could argue that the differing results might be due to the different stimulus modalities used in these two studies: Whereas Koch, Frings, and Schuch used visual stimuli, we employed auditory stimuli in our first experiment and added visual stimuli in our second experiment. However, RR effects have been replicated in various studies and seem to be generalizable to the use of various stimuli. Specifically, Seibold et al. (2019) found evidence that RR effects arise independently of the stimulus modality. Thus, we do not believe that the present lack of episodic influence on the pattern of RR effects was due to the use of auditory stimuli. Furthermore, this dissociation of response-irrelevant episodic features from the response-associated binding remained present even when stimulus modality repetition trials for the auditory or visual stimulus modality were analyzed separately. Further minor differences between our experimental setup and that in Koch, Frings, and Schuch’s experiments include the use of more salient visual cues in the present study (cue heights: 6.3 vs. 1.0 cm,) as well as the use of more discriminable response keys in Koch, Frings, and Schuch’s experiments (the separate keys “A” and “#” vs. the adjacent keys “V” and “B”). Although we believe that these differences could result in facilitated response selection, we do not believe that they specifically affected the interaction of task switches and response switches, especially since recent evidence from Seibold et al. (2019) suggests that enhanced response discriminability does not alter the pattern of RR effects. It is conceivable that the contextual effects on RR effects that were found in the preceding study employing visual stimuli (Koch, Frings, & Schuch, 2018) are less robust than has been presumed and do not necessarily generalize to other modalities. For further research, it will be necessary to profoundly explore modality effects before deriving theoretical conclusions.
The priming and inhibition account (e.g., Hübner & Druey, 2006) might offer an explanation for why RR benefits remain unaffected by cue modality or stimulus modality switches. According to this account, RR benefits are supposed to originate from positive stimulus category priming rather than from episodic binding. It is suggested that the persisting activation of a modality-unspecific abstract stimulus category (e.g., “numbers smaller than 5” in a magnitude task) triggers the beneficial effect of a response repetition in task repetitions. However, the mechanism of modality-unspecific priming of abstract stimulus categories, in turn, does not offer a viable explanation for the higher (modality-specific) task switch costs that we consistently found in two experiments for cue modality repetitions (Exp. 1) and stimulus modality repetitions (Exp. 2), and that were also found by Koch, Frings, and Schuch (2018). Yet, in task switches, the absent modulation of RR costs by cue modality switches or stimulus modality switches might suggest that RR costs in task switches originate from inhibition automatically following response execution, in line with the dual-mechanism account (Koch, Frings, & Schuch, 2018) as well as with the priming and inhibition account (e.g., Hübner & Druey, 2006).
The reconfiguration account (Kleinsorge & Heuer, 1999) offers another perspective on RR effects: Whereas RR benefits are explained by modality-unspecific positive stimulus category priming, RR costs are explained by a hierarchically organized task-set control mechanism, composed of a superordinate task level implementing stimulus categorization and a subordinate response level implementing response selection. Task switches are presumed to automatically trigger a reconfiguration of the subordinate response, but not vice versa. Consequently, in the case of a task switch, a response switch is automatically initiated but has to be “switched back” when the response actually repeats, producing RR costs. Recent evidence by Korb, Jiang, King, and Egner (2017) underpins this theoretical account by pointing out that task-set control and response selection are implemented in different brain areas. Using neuroimaging (functional magnetic resonance imaging data) and noninvasive neurostimulation (transcranial magnetic stimulation data), the authors found evidence that the proposed hierarchically organized task-set control mechanism can be observed in the neurocognitive architecture: Activation in the presupplementary motor area (preSMA) was found to be associated with task-set control processes, whereas activation in the supplementary motor area (SMA) was found to be associated with response-set control processes. Furthermore, the authors found evidence for interaction of both structures with the basal ganglia. Importantly, they propose a unidirectional constraint of the preSMA–basal ganglia loop, representing task selection, on the SMA–basal ganglia loop, representing response selection, thus underpinning the hierarchical organization of the superordinate task level and the subordinate response level. Regarding the present results, the binding of the cue modality and stimulus modality at the level of the task is compatible with the neurocognitive architecture explained by Korb et al. Furthermore, the dissociation of the response from such a binding can be explained by dissociable neurocognitive structures representing the task-set reconfiguration and the response selection.
Conclusion
In the present study, we replicated the typical cost–benefit pattern of response-repetition effects in task switching, using an experimental setup with auditory and visual cues and stimuli. We found evidence for the episodic binding of the cue modality and the stimulus modality to the task. At the same time, response-repetition effects were not modulated by cue modality switches or stimulus modality switches, meaning that in our two experiments, RR benefits as well as RR costs were independent of the cue modality and the stimulus modality. We suggest that RR effects are elicited by response-associated bindings, which are not necessarily affected by all episodic task features to the same extent. Although some task-relevant features (such as the stimulus category and the given response) are strongly represented in episodic bindings, some other, task-irrelevant features of the task episode (such as the cue modality or the stimulus modality) may only be included in the binding with a nonsignificant weight. Further research will be required in order to explore which boundary conditions determine whether episodic task features are integrated in response-associated bindings. Notably, our results are also compatible with the reconfiguration account proposed by Kleinsorge and Heuer (1999), implying a hierarchically organized task-set control mechanism. The cue modality and stimulus modality could wield influence on the superordinate task level, whereas the subordinate response level is independent of the contextual overlap between task episodes.
Notes
Following up on Koch, Frings, and Schuch (2018), we employed two cue modalities (high vs. low cue via the auditory vs. visual modality) and two tasks (magnitude vs. parity task) to isolate cue modality switch costs from task switch costs. By employing this setup, a repeated cue modality could be combined with a repeated or switched task (for a discussion, see Koch, Frings, & Schuch, 2018).
There are two possibilities to control for stimulus-repetition effects. One possibility is to exclude stimulus repetitions from the data analysis (as was done in the present study). Another possibility is to exclude stimulus repetitions from occurring in the experiment (as was done in the studies by Schuch & Koch, 2004, 2006, 2010). In the latter studies, stimulus category repetitions and switches occurred with a 1:1 ratio, whereas immediate stimulus repetitions were excluded by design.
The present results remain robust when z-scores are computed separately for each participant in each condition. Here, outliers were defined by z-transforming the RTs separately for each participant averaged over conditions, to ensure the comparability of our results to those from the preceding study by Koch, Frings, and Schuch (2018).
References
Altmann, E. M. (2011). Testing probability matching and episodic retrieval accounts of response repetition effects in task switching. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37, 935–951. https://doi.org/10.1037/a0022931
Dehaene, S., Bossini, S., & Giraux, P. (1993). The mental representation of parity and number magnitude. Journal of Experimental Psychology: General, 122, 371–396. https://doi.org/10.1037/0096-3445.122.3.371
Druey, M. D. (2014). Stimulus-category and response-repetitions effects in task switching: An evaluation of four explanations. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40, 125–146. https://doi.org/10.1037/a0033868
Druey, M. D., & Hübner, R. (2008). Response inhibition under task switching: Its strength depends on the amount of task-irrelevant response activation. Psychological Research, 72, 515–527. https://doi.org/10.1007/s00426-007-0127-1
Frings, C. (2011). On the decay of distractor-response episodes. Experimental Psychology, 58, 125–131. https://doi.org/10.1027/1618-3169/a000077
Frings, C., Rothermund, K., & Wentura, D. (2007). Distractor repetitions retrieve previous responses to targets. Quarterly Journal of Experimental Psychology, 60, 1367–1377. https://doi.org/10.1080/17470210600955645
Frings, C., Schneider K. K., & Moeller, B. (2014). Auditory distractor processing in sequential selection tasks. Psychological Research, 78, 411–422. https://doi.org/10.1007/s00426-013-0527-3
Gade, M., Schuch, S., Druey, M. D., & Koch, I. (2014). Inhibitory control in task switching. In J. Grange & G. H. Houghton (Eds.), Task switching and cognitive control (pp. 137–159). New York, NY: Oxford University Press. https://doi.org/10.1093/acprof:osobl/9780199921959.003.0007
Grzyb, K. R., & Hübner, R. (2012). Response-repetition costs in task switching: How they are modulated by previous-trial response-category activation. Acta Psychologica, 139, 97–103. https://doi.org/10.1016/j.actpsy.2011.10.006
Hommel, B., Müsseler, J., Aschersleben, G., & Prinz, W. (2001). The Theory of Event Coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences, 24, 849–878, disc. 878–937. https://doi.org/10.1017/S0140525X01000103
Hübner, R., & Druey, M. D. (2006). Response execution, selection, or activation: what is sufficient for response-related repetition effects under task shifting? Psychological Research, 70, 245–261. https://doi.org/10.1007/s00426-005-0219-8
Jersild, A. T. (1927). Mental set and shift. Archives of Psychology, 14(Whole No. 89), 5–82.
Jost, K., De Baene, W., Koch, I., & Brass, M. (2013). A review of the role of cue processing in task switching. Zeitschrift für Psychologie, 221, 5–14. https://doi.org/10.1027/2151-2604/a000125
Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Philipp, A. M., & Koch, I (2010). Control and interference in task switching—A review. Psychological Bulletin, 136, 849–874. https://doi.org/10.1037/a0019842
Kleinsorge, T. (1999). Response repetition benefits and costs. Acta Psychologica, 103, 295–310. https://doi.org/10.1016/S0001-6918(99)00047-5
Kleinsorge, T., & Heuer, H. (1999). Hierarchical switching in a multi-dimensional task space. Psychological Research, 62, 300–312. https://doi.org/10.1007/s004260050060
Koch, I., Frings, C., & Schuch, S. (2018). Explaining response-repetition effects in task switching: Evidence from switching cue modality suggests episodic binding and response inhibition. Psychological Research, 82, 570–579. https://doi.org/10.1007/s00426-017-0847-9
Koch, I., Poljac, E., Müller, H., & Kiesel, A. (2018). Cognitive structure, flexibility, and plasticity in human multitasking—An integrative review of dual-task and task-switching research. Psychological Bulletin, 144, 557–583. https://doi.org/10.1037/bul0000144
Koch, I., Schuch, S., Vu, K.-P. L., & Proctor, R. W. (2011). Response-repetition effects in task switching—Dissociating effects of anatomical and spatial response discriminability. Acta Psychologica, 136, 399–404. https://doi.org/10.1016/j.actpsy.2011.01.006
Korb, F. M., Jiang, J., King, J. A., & Egner, T. (2017). Hierarchically organized medial frontal cortex–basal ganglia loops selectively control task- and response-selection. Journal of Neuroscience, 37, 7893–7905. https://doi.org/10.1523/JNEUROSCI.3289-16.2017
Lukas, S., Philipp, A. M., & Koch, I. (2014). Crossmodal attention switching: Auditory dominance in temporal discrimination tasks. Acta Psychologica, 153, 139–146. https://doi.org/10.1016/j.actpsy.2014.10.003
Meiran, N. (2000). Modeling cognitive control in task-switching. Psychological Research, 63, 234–249. https://doi.org/10.1007/s004269900004
Memelink, J., & Hommel, B. (2013). Intentional weighting: a basic principle in cognitive control. Psychological Research, 77, 249–259. https://doi.org/10.1007/s00426-012-0435-y
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. https://doi.org/10.1016/S1364-6613(03)00028-7
Peirce, J. W. (2009). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2, 10. https://doi.org/10.3389/neuro.11.010.2008
Quinlan, P. T. (1999). Sequential effects in auditory choice reaction time tasks. Psychonomic Bulletin & Review, 6, 297–303. https://doi.org/10.3758/BF03212333
Quinlan, P. T., & Hill, N. I. (1999). Sequential effects in rudimentary auditory and visual tasks. Perception & Psychophysics, 61, 375–384. https://doi.org/10.3758/BF03206894
Rogers, R. D., & Monsell, S. (1995). Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124, 207–231. https://doi.org/10.1037/0096-3445.124.2.207
Rouder, J. N., Speckman, P. L., Sun, D., Morey, R. D., & Iverson, G. (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16, 225–237. https://doi.org/10.3758/PBR.16.2.225
Schuch, S., & Koch, I. (2004). The costs of changing the representation of action: Response repetition and response–response compatibility in dual tasks. Journal of Experimental Psychology: Human Perception and Performance, 30, 566–582. https://doi.org/10.1037/0096-1523.30.3.566
Schuch, S., & Koch, I. (2006). Task switching and action sequencing. Psychological Research, 70, 526–540. https://doi.org/10.1007/s00426-005-0014-6
Schuch, S., & Koch, I. (2010). Response-repetition effects in task switching with and without response execution. Acta Psychologica, 135, 302–309. https://doi.org/10.1016/j.actpsy.2010.07.016
Seibold, J. C., Koch, I., Nolden, S., Proctor, R. W., Vu, K.-P. L., Schuch, S. (2019). Response repetitions in auditory task switching: The influence of spatial response distance and of the response–stimulus interval. Acta Psychologica, 199, 102875. https://doi.org/10.1016/j.actpsy.2019.102875
Spence, C., & Driver, J. (1997). On measuring selective attention to an expected modality. Perception & Psychophysics, 59, 389–403. https://doi.org/10.3758/BF03211906
Steinhauser, M., Hübner, R., & Druey, M. D. (2009). Adaptive control of response preparedness in task switching. Neuropsychologia, 47, 1826–1835. https://doi.org/10.1016/j.neuropsychologia.2009.02.022
Vandierendonck, A., Liefooghe, B., & Verbruggen, F. (2010). Task switching: Interplay of reconfiguration and interference control. Psychological Bulletin, 136, 601–626. https://doi.org/10.1037/a0019791
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kandalowski, S.R.M., Seibold, J.C., Schuch, S. et al. Examining binding effects on task switch costs and response-repetition effects: Variations of the cue modality and stimulus modality in task switching. Atten Percept Psychophys 82, 1632–1643 (2020). https://doi.org/10.3758/s13414-019-01931-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-019-01931-0