Abstract
Odors can be perceived as arising from the environment or as part of a flavor located in the mouth. One factor that may dictate where an odor is perceived to be is concurrent gustatory stimulation in the mouth. A taste may impair the ability to attend to an odor, especially if they are perceptually similar. Alternatively, salient mouth-based features of a flavor might command attention at the expense of smell. Experiment 1 and 2, using different stimulus sets, explored the impact of perceptually similar and dissimilar pairings of tastes in the mouth and odors at the nose. In each case, these were followed by judgments of the odor’s location (mouth vs. nose). Perceptual similarity had no impact on localization judgments. Experiment 3 then manipulated the salience of the olfactory and gustatory cues and showed that each could independently shift the perceived location of an odorant—salient olfactory cues toward the nose and gustatory cues toward the mouth. These findings suggest that the salient features of a flavor may command attention at the expense of olfaction and, thereby, contribute to oral localization, with implications for flavor binding.
Similar content being viewed by others
Introduction
When an odor is sniffed via the external nares (orthonasally), it is perceived as coming from the environment. During eating and drinking, volatiles ascend via the internal nares (retronasally), stimulating the same set of receptors as those used during orthonasal perception. However, in this case, olfaction contributes to a flavor percept (i.e., the interaction among taste, smell, and somatosensation), which is perceived as coming from the mouth (Rozin, 1982). The mechanism(s) responsible for the localization of olfactory experience to the internal (mouth) or external (nose) environment are not well understood, but two basic processes have been suggested. One is that localization is dictated by the direction of nasal airflow, which differs between the orthonasal (inhalation) and retronasal (exhalation) routes (Hummel et al., 2006; Small, Gerber, Mak, & Hummel, 2005). This difference may be detected by the nasal mucosa (e.g., J. W. Scott, Acevedo, Sherrill, & Phan, 2007) and/or by the differential sensitivity of the posterior and anterior nares to mechanical and irritant stimulation (Frasnelli, Heilmann, & Hummel, 2004). A second process concerns the contribution of events in the mouth to oral localization (e.g., Green, 2002; Murphy & Cain, 1980).
Participants are not generally aware that smell is a major contributor to a food’s flavor. Several lines of evidence support this claim, including the observation that of all of the major languages surveyed, none had a specific lay term for the olfactory component of flavor (Rozin, 1982). Relatedly, when people lose just their sense of smell, this is often initially reported as a loss of smell and taste (e.g., Deems et al., 1991). For these reasons, it may be inaccurate to speak off localization of an odor to the mouth (although it is referred to here as such for brevity), because under the circumstances where this occurs, participants may simply not know that an odor is present. Instead, there are at least two possibilities that should be considered. First, it may be more appropriate to regard olfactory localization as reflecting the degree of endogenous (i.e., voluntary; see Corbetta & Shulman, 2002; Posner, 1980) attention that can be directed toward the olfactory channel. This might vary from high (sniffing/orthonasal) to low (flavor/retronasal). An alternative way of stating this same possibility is that oral localization of a smell may depend on the degree to which it can be distinguished from the taste component, such that greater perceptual similarity may make it harder to voluntarily attend to the olfactory channel. A second possibility is that oral localization may depend on exogenous—involuntary—attention (see Corbetta & Shulman, 2002; Posner, 1980). Tastes may be perceived as more intense sensations, they may be pungent (i.e., activate the oral trigeminal system), and they may be strongly liked or disliked. Tastes, then, could be typically more salient and attention-demanding stimuli than smells. As long as taste fulfils this role, attention may remain captured by this modality and, thus, spatially directed to the mouth, at the expense of olfaction.
Irrespective of the precise nature of this process, the apparent location of an odor can be affected by events within the mouth. In a previous series of experiments, Stevenson, Oaten, and Mahmut (in press), examined the role of oral stimulation in generating olfactory localization to the mouth. Using a task based on von Bekesy’s (1964) work, participants were asked to sniff solutions (some odorized and some not) while different somatosensory or gustatory/somatosensory stimuli were present in their mouths. Their task was to judge how certain they were that the smell was coming from the jar they were sniffing or the fluid in their mouth. It had previously been suggested that oral somatosensory cues might be important in generating olfactory oral localization (Green, 2002; Murphy & Cain, 1980), but our work suggested that somatosensation alone is insufficient. Instead, a taste was needed (i.e., gustatory and somatosensory stimulation; Stevenson et al., in press).
That tastes might be important in generating oral localization is perhaps anticipated by a number of other findings. As we noted above, naïve participants do not readily recognize the olfactory component of flavor, often referring to taste rather than correctly to taste and smell. Note here that it is not somatosensory stimulation that is substituted for smell but taste, suggesting a close association between these modalities. A further set of findings indicate that olfactory perception can be affected by taste and that taste perception can be affected by smell (e.g., Davidson, Linforth, Hollowood, & Taylor, 1999; Frank & Byram, 1988; Von Sydow, Moskowitz, Jacobs, & Meiselman, 1974). Odors are routinely described as having tastelike properties, especially sweetness, and when such odors are added to a sweet solution and sampled by mouth, this results in participants’ judging the combination as sweeter than sucrose alone—taste enhancement (e.g., Stevenson, Prescott, & Boakes, 1999). These interactions between perceptually similar tastes and smells extend well beyond self-report. Sweet tastes facilitate the detection of sweet smells (e.g., Dalton, Doolittle, Nagata, & Breslin, 2000), sweet smells facilitate the recognition of sweet tastes (White & Prescott, 2007), and sweet smells interfere with memory for sweet tastes (Stevenson & Oaten, 2010).
Interactions between tastes and smells may reflect two distinct components (Valentin, Chrea, & Nguyen, 2006). The first is a general tendency to integrate concurrently experienced tastes and smells, irrespective of experience. This can be seen, most clearly, in the taste enhancement effect. Valentin et al. found that combinations of tastes and smells (all food-related odors) tended to result in enhancement effects irrespective of their shared tastelike characteristics (and see Clark & Lawless, 1994). That is, any combination of taste- and food-related smell may result in increases in taste-related judgments, even if that combination is not usually experienced together. However, under conditions in which a more detailed rating set is provided (e.g., sweet and strawberry ratings for a strawberry odor–sucrose mixture), taste–smell interactions tend to disappear for perceptually dissimilar mixtures (i.e., where the tastelike qualities of the odor do not match the physically present taste) but persist for perceptually similar mixtures (see Valentin et al., 2006; Van der Klaauw & Frank, 1996). There may, then, be a general tendency to integrate taste and smell in the mouth, as well as a more specific tendency to integrate tastes and smells, which share common perceptual qualities.
This apparent distinction between a general and a specific process would seem to have a bearing on the mechanisms that may govern taste-driven oral localization of odors (see the second paragraph). In one account above, we suggested that the perceived location of an odor might depend on the capacity to voluntarily attend to it—with reduced capacity to attend equating with greater oral localization (i.e., the participant is simply unable to tell whether he or she is perceiving an odor or a taste). If the capacity to voluntarily attend to an odor is impaired by perceptual similarity (i.e., the taste and smell are less discriminable), greater similarity should be associated with greater oral localization. Thus, localization for perceptually similar tastes and smells should reflect both the general and specific forms of integration, and oral capture here should exceed that of perceptually dissimilar tastes and smells (i.e., the taste and smell should be more discriminable in this case), where only the general form of integration should occur.
The second account above suggested that attention might be drawn automatically to salient features of the flavor and that, since tastes are often the most salient feature, they would thus attract attention at the expense of the olfactory channel. In this case, what may be important is the degree to which particular features are attention demanding. This could include unpleasantness, since affectively negative stimuli are more likely to command attention than are affectively positive stimuli (e.g., Rozin & Royzman, 2001). If an odor, then, is particularly unpleasant (relative to the taste), but also if it is novel, intense, or pungent (relative to the taste)—that is, any salient feature—this should serve to attract attention to the olfactory channel, with a reduction (or perhaps loss) of oral localization. This account would suggest that perceptual similarity is of little importance, because it is the relative salience of the flavor’s features that dictates attentional capture. The aim of the three experiments reported here was to assess the role of perceptual similarity (Experiment 1 and 2) and feature salience (Experiment 3) in oral localization of odors and, thus, determine which of these two accounts is best supported.
Experiment 1
In Experiment 1, we examined the impact of perceptual similarity on taste-driven odor localization. The procedure here was developed in a preceding series of experiments, in which all odors were presented orthonasally and tastes (and other oral stimuli) were presented to the mouth (Stevenson et al., in press). Participants were asked, in each case, to indicate how certain they were that the odor came from the fluid being sniffed at the nose or from the fluid introduced into their mouth. The present experiment used the same technique but manipulated the perceptual similarity of the stimuli by using all four possible pairings between a sweet and a nonsweet taste versus a sweet and a nonsweet food odor. In addition to including perceptually similar and dissimilar trials, a number of other trial types were included. First, both odors (sweet and nonsweet) were presented when water was in the mouth, and with no oral stimulus present. These two conditions served to ensure that we could replicate our previous observation that the addition of a taste acts to increase oral localization and, in the case of the no-oral-stimulus condition, to show that the odor here was correctly attributed to the external environment. Second, all of the four oral (mouth) conditions—none, water, sweet, and nonsweet taste—were fully crossed with both odors and also with an equal number of dummy water blanks. The water blanks served to ensure that at least half the trials contained no olfactory component, thereby minimizing adaptation. Finally, at the end of the experiment, we asked participants to evaluate both odors and tastes on a series of category-rating scales, to check their degree of perceptual similarity.
Method
Participants
Sixteen healthy participants (mean age = 22.8 years, SD = 4.7) took part in Experiment 1 for a small cash payment. No participant had taken part in a related study, as with the other experiment reported here.
Stimuli
The two target odorants were vanilla (4 g/L; Dragoco, Sydney) and a nonsweet-smelling food-based odor, Oolong tea (5 g/L; Quest, Sydney), presented in aliquots of 20 ml in transparent 250-ml screw-top jars. The two tastants were sucrose (50 g/L; Sigma-Aldrich, Sydney) and citric acid (1.16 g/L; Sigma-Aldrich, Sydney), presented as 10-ml samples in transparent disposable 20-ml cups. All the solutions were transparent and visually indistinguishable.
Procedure
It was explained to participants that their task was to judge the source (i.e., our proxy measure of its perceived location) of an odor. Participants exhaled and then poured a solution into their mouths (if this was required on a particular trial) with their nosees pinched to prevent ortho- and retronasal olfaction. Once the taste or water was placed in the mouth, participants were instructed to unpinch their noses and sniff (via inhalation) the contents of a jar. They were then asked to pinch shut their noses again, exhale through the mouths, and rate the “location” of the odor on a 7-point category scale (from jar [1] to unsure [4] to mouth [7]). Participants then expectorated if a solution was present, rinsed with water, and expectorated, and only then were they instructed to unpinch their noses. This constituted a trial.
Participants were tested individually, and the experimenter directed their actions on every trial, so as to ensure compliance with the procedure. Prior to the experimental trials, participants received a detailed training session. This started with an explanation of the rating scale. Participants were instructed to rate 1 (jar) if they were very certain the odor came from the jar, 2 or 3 if they were less certain, 4 if they were unsure, 5 or 6 if they thought it was in their mouths (i.e., from the cup) but were less certain, and 7 if they were very certain it was in their mouths. This was followed by a series of practice trials on which the aim was to show the participant how to pinch and unpinch their noses. This manoeuvre was then repeated, but this time with water in their mouths. If the participants could accomplish this correctly, they then ran through two practice trials using water in the cup (i.e., mouth) and water in the jar (i.e., at the nose). If a procedural error occurred on either practice trial, this was corrected, and an additional practice trial was undertaken until two error-free trials were completed.
In total, there were 16 experimental trials, which were presented in a different randomized order for each participant. Each trial was separated by a 60-sec intertrial interval to further minimize fatigue. There were four types of stimuli presented in the cup: no fluid at all (the participants just being asked to close their mouths), water, sucrose, and citric acid solution. There were three types of sniffed stimulus: water, vanilla, and oolong tea. While the vanilla and oolong trials were fully crossed with the four types of stimuli presented in the cup (giving four trials with each odor, or eight trials in total), dummy trials with water were presented twice as frequently at the nose for each type of stimulus presented in the cup (giving eight trials in total).
Finally, participants undertook two further judgment tasks in counterbalanced order. On the odor judgment task, they evaluated the vanilla and oolong tea odors. Each solution was sniffed three times, after which participants rated the stimulus, using five 7-point category rating scales: how foodlike, strong, sweet, and sour it smelled (from 1 [not at all] to 7 [very]), and how much they liked or disliked it (from 1 [dislike], to 4 [indifferent], to 7 [like]). On the taste judgment task, they evaluated sucrose and citric acid, swilling and spitting each solution, and then rating each taste for sweetness, sourness, strength, and liking (scales as above). The whole procedure took around 1 hr to complete.
Analysis
For clarity, stimuli presented in the jar are referred to as nose stimuli, and stimuli presented in the cup are referred to as mouth stimuli. For the nose stimuli, ratings completed for the dummy water on the nose trials were not analyzed, since these had little bearing on the odor-related similarity effects, which were of primary interest here.
Results
Odor and taste ratings
Participants rated vanilla as smelling sweeter (M = 5.9) than oolong tea (M = 3.2), t(15) = 5.49, p < .001, and oolong tea as sourer (M = 2.8) than vanilla (M = 1.4), t(15) = 3.00, p < .01. Vanilla was rated as being liked more (M = 5.9) than oolong tea (M = 3.4), t(15) = 6.85, p < .001, and vanilla was also judged to be more foodlike (M = 5.3) than oolong tea (M = 2.9), t(15) = 4.12, p < .001. There was no significant difference in intensity between these two odors.
Sucrose was judged to taste sweeter (M = 5.7), t(15) = 6.74, p < .001, than citric acid (M = 1.6), and citric acid was judged to taste sourer (M = 5.7), t(15) = 10.37, p < .001, than sucrose (M = 1.1). Citric acid was also judged as more intense (M = 6.1), t(15) = 4.44, p < 0.001, than sucrose (M = 4.9), and citric acid was liked less (M = 2.2), t(15) = 7.68, p < 0.001, than sucrose (M = 5.1).
To establish the degree of perceptual similarity for each odor–taste pair, we calculated the sum of the absolute difference between the odor and taste’s similar feature and dissimilar feature (e.g., for \( {\hbox{oolong}}--{\hbox{citric}}\;{\hbox{acid}} = \left( {|{\hbox{oolong sourness}}--{\hbox{citric acid sourness}}|} \right) + \left( {|{\hbox{oolong sweetness}}--{\hbox{citric acid sweetness}}|} \right) \). Here, a low value indicated high similarity, and a higher value indicated greater dissimilarity. While the vanilla–citric acid (M = 8.6) and vanilla–sucrose (M = 1.6) pairs differed as expected, t(15) = 6.35, p < .001, the oolong–citric acid (M = 5.3) and oolong–sucrose (M = 4.3) did not (t < 1). So, while the vanilla pairs clearly represented perceptually similar and dissimilar combinations, the oolong pairs did not.
Localization ratings
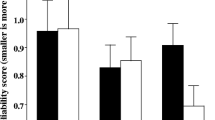
Figure 1 presents mean localization data for the two odors for each mouth condition. Before examining for any similarity-related effects, we tested whether localization for both odors combined was greater when the two taste stimuli were present, relative to when water was in the mouth or there was no oral stimulus (using a Bonferroni corrected α = .017). Reports of localization did not significantly differ when water was in the mouth (M = 1.5), relative to no oral stimulus (M = 1.2). However, the presence of a tastant generated significantly more localization toward the mouth—or alternately, away from the nose—(M = 3.3), relative to water, t(15) = 4.32, p < .017, and to no oral stimulus, t(15) = 5.78, p < .017. In addition, the number of participants reporting localization to the mouth was also examined (i.e., a score of 5 or more). When water was in the mouth or when there was no oral stimulus, no participant reported oral localization on any trial, but when a taste was present, 12/16 demonstrated localization on one or more trials, and this difference was significant [McNemar change test, χ2(1) = 10.08, p < .002]. Thus, consistent with our previous findings, tastants were able to generate greater oral localization, relative to water alone and to the no-oral-stimulus condition.
Mean localization ratings (and SEs) for each nose (stimulus presented for sniffing) and mouth (stimulus presented for tasting) condition in Experiment 1
We then tested the magnitude of the localization effect between the vanilla pairs, which significantly differed in perceptual similarity, and for the oolong pairs, which did not. There was no significant difference between the vanilla pair, with localization being equivalent for the vanilla–sucrose (M = 3.3) and vanilla–citric acid (M = 3.3) conditions. However, for the oolong pairs, there was greater localization in the oolong–citric acid condition (M = 4.0) than in the oolong–sucrose condition (M = 2.6), t(15) = 4.07, p < .001, even though they did not differ in perceptual similarity.
We also examined whether the number of participants demonstrating oral localization (i.e., a score greater than 5) differed between the vanilla and oolong pairs. For vanilla pairs, there was no significant difference between the sucrose (3/16) and citric acid (5/16) pairings. However, for the oolong pairs, there were a greater number of instances of oral localization for citric acid (9/16), relative to sucrose (1/16) [McNemar change test, χ2 (1) = 6.13, p < .02].
Relationship between odor and localization variables
In an attempt to determine whether differences in the salience of taste and smell components might be important in dictating oral capture for the oolong tea pairs, we examined the variables that might predict this difference in localization between oolong tea in sucrose minus oolong tea in citric acid. Since sample size was small for regression, we collapsed odor variables (for oolong tea) that were significantly related, producing three variables: a hedonic measure (hedonic rating and foodlikeness), a quality measure (sweetness minus sourness), and intensity. These three predictors were entered simultaneously, with backward elimination, and the final model was significant, F(1, 14) = 10.27, p < .01, accounting for 38% of the variance (adjusted R 2). Only one predictor was included in this final model, the hedonic variable, indicating that the more pleasant and foodlike oolong tea was judged to be, the smaller the difference in localization between citric acid and sucrose conditions. The same approach was then adopted for the difference in localization between vanilla in sucrose minus vanilla in citric acid. None of the three variables, drawn from the evaluations of the vanilla odor, significantly predicted differences in localization responses.
Hedonic data
Finally, we examined whether the hedonic ratings for each taste and smell bore the same relationship across the four odor–taste combinations. We calculated the difference score for each combination between the hedonic rating for the odor minus the hedonic rating for the taste. In particular, we were interested here in whether odor hedonic ratings would be more negative (i.e., salient and attention demanding) than those for any taste, within each pair. Consequently, we conducted one-sample t tests (with Bonferroni-adjusted α = .0125). The taste was judged as liked significantly less than the odor for vanilla–sucrose (M = 0.9), t(15) = 3.66, p < .0125, vanilla–citric acid (M = 3.8), t(15) = 9.82, p < .0125, and oolong–citric acid (M = 1.3), t(15) = 3.10, p < .0125; however, this relationship was reversed for oolong–sucrose (M = –1.6), t(15) = 3.99, p < .0125—the combination that revealed the least oral localization.
Discussion
While the vanilla pairings—vanilla–sucrose and vanilla–citric acid—represented perceptually similar and dissimilar pairings, respectively, there was no evidence that this difference impacted on localization ratings. However, for the oolong pairings, which did not significantly differ in perceptual similarity, we observed that oral localization differed between the odor pairs, being higher for the oolong–citric acid pairing than for the oolong–sucrose pairing. Regression indicated that it was hedonic discordance that best accounted for this difference, and the oolong–sucrose condition was the only pair in which the odor was disliked more than the taste. These findings would seem to suggest that perceptual similarity is not an important factor in dictating oral localization. However, it is possible that our findings for vanilla are somehow atypical and that other similar/dissimilar odor–taste pairs might behave differently. For this reason we conducted a second experiment, using two further odors, where we attempted to manipulate perceptual similarity more successfully for both pairs than we had in Experiment 1.
Experiment 2
The principal aim of Experiment 2 was to determine whether effects of perceptual similarity and dissimilarity on localization could be obtained using a new set of stimuli. A perceptual similarity measure was also included here to further ensure that the stimuli were appropriately similar/dissimilar, as expected. In addition, we included a measure of odor intensity after each localization rating to test whether perceptually similar odor taste pairs would be judged to have a more intense smell than the target odor presented in the absence of a taste (i.e., with water in the mouth). On the basis of the finding that sweet tastes tend to be judged as tasting sweeter when sweet smells are added (e.g., Stevenson et al., 1999) and sweet smells are judged as more intense in sucrose (e.g., Davidson et al., 1999; von Sydow et al., 1974), we expected, in particular, that the sweet-smelling odor–taste pair would evidence enhancement, demonstrating an effect of perceptual similarity within our procedure.
Method
Participants
Seventeen healthy participants (mean age = 22.6 years, SD = 4.3) took part for a small cash payment.
Stimuli
The two target odorants were plum (0.13 g/L; Quest, Australia) and a non-sweet-smelling odor oregano (0.18 g/L; Dragoco, Australia), presented in the same manner as in Experiment 1. Tastants were sucrose (50 g/L; Sigma-Aldrich, Sydney) and sodium chloride (4.1 g/L; Sigma-Aldrich, Sydney), again presented in the same manner as in Experiment 1. All the solutions were transparent and visually indistinguishable.
Procedure
The procedure was identical to that in Experiment 1 in all but two ways. First, in addition to obtaining localization ratings, participants were also asked now to rate how intense the odor smelled, using a 7-point category rating scale (from not at all [1] to very [7]). Second, after completing the odor and taste evaluations (in counterbalanced order), participants were presented with four pairs of stimuli, with order of presentation counterbalanced across participants. The four pairs of stimuli were all composed of a taste in a cup (sucrose or saline) and a smell in a jar (plum or oregano). Participants’ task, on two of these trials, was to sample the taste, rinse, then sniff the odor orthonasally, and then judge how similar the two stimuli were. On the other two trials, the odor was smelled first, and the taste was sampled second. In each case, participants were then asked to judge their similarity on a 7-point category scale (from not at all similar [1] to very similar [7]).
Results
Ratings and similarity scores
Participants rated plum as smelling sweeter (M = 6.2) than oregano (M = 1.9), t(16) = 12.15, p < .001, and oregano as smelling saltier (M = 4.5) than plum (M = 1.4), t(16) = 7.36, p < .01. Plum was also rated as being liked more (M = 5.9) than oregano (M = 4.4), t(16) = 3.36, p < .005, but there were no significant differences between these odors in foodlikeness or intensity.
Sucrose was judged to taste sweeter (M = 6.3), t(16) = 21.58, p < .001, than saline (M = 1.3), and saline was judged to taste saltier (M = 6.2), t(16) = 25.22, p < .001, than sucrose (M = 1.1). Sucrose and saline were judged as equally intense, but saline was liked less (M = 3.2), t(16) = 6.93, p < .001, than sucrose (M = 5.7).
The same measure of perceptual similarity as that developed for Experiment 1 was also employed here, with a lower value indicating higher similarity and a higher value indicating lower similarity. The plum–saline (M = 9.7) and plum–sucrose (M = 0.9) pairs significantly differed, t(16) = 15.71, p < .001, as did the oregano–saline (M = 2.7) and oregano–sucrose pairs (M = 7.8), t(16) = 4.69, p < .001.
The similarity ratings from the direct comparison of the two odors and tastes were also examined, revealing the same picture as the other similarity index. Plum was judged to smell more similar to sucrose (M = 5.6), relative to saline (M = 1.9), t(16) = 13.39, p < .001, and oregano was judged to smell more similar to saline (M = 4.8), relative to sucrose (M = 1.5), t(16) = 5.36, p < .001.
Localization ratings
Figure 2 presents mean localization data for the two odors in each mouth condition. Before examining for any congruency-related effects, we again tested whether oral localization for both odors combined was greater when the two taste stimuli were present, relative to when water was in the mouth or there was no oral stimulus (using a Bonferroni-corrected α = .017). Localization did not significantly differ when water was in the mouth (M = 1.6), relative to no oral stimulus (M = 1.3). However, the presence of a tastant generated significantly more localization toward the mouth (M = 2.3)—or away from the nose—relative to water, t(16) = 3.64, p < .017, and to the no-oral-stimulus condition, t(16) = 4.79, p < .017. In addition, the number of participants reporting localization to the mouth was examined (i.e., a score of 5 or more). When water was in the mouth or when there was no oral stimulus, 2/17 participants reported oral localization on any trial, but when a taste was present, 9/17 demonstrated localization on one or more trials, and this difference was significant [McNemar change test, χ2(1) = 4.50, p < .05]. So, as in Experiment 1, tastants were able to generate more oral localization.
Mean localization ratings (and SEs) for each nose (stimulus presented for sniffing) and mouth (stimulus presented for tasting) condition in Experiment 2
We then tested the magnitude of the localization effect in the same manner as in Experiment 1. There was no significant difference in localization between the plum pairs (plum–sucrose, M = 3.2, vs. plum–saline, M = 2.9), t < 1, or between the oregano pairs (oregano–saline, M = 3.2, vs. oregano–sucrose, M = 2.5), t < 1.6. We also examined whether the number of participants demonstrating oral localization (i.e., a score greater than 5) differed within the plum pairs and the oregano pairs. There were no differences within either set of pairs, with 5/17 participants demonstrating oral localization with plum–sucrose, 5/17 with plum–saline, 4/17 with oregano–saline, and 2/17 with oregano–sucrose.
Intensity ratings
Intensity ratings for both odors in the different mouth conditions are presented in Table 1. Relative to the odor sniffed when water was in the mouth, only the plum–sucrose combination was judged to smell more intense (M diff = 0.7), t(16) = 3.17, p < .01, with no significant differences observed for the other three conditions.
Relationship between odor and localization variables
We used the same regression approach as in Experiment 1 to explore whether the odor evaluations were predictive of the degree of localization between similar and dissimilar odor–taste pairs. Odor ratings were collapsed in the same manner as in Experiment 1, and in addition, the difference in similarity ratings for each respective odor with the congruent and incongruent tastes was also included. The four predictors—hedonics, taste quality, intensity, and similarity—were entered simultaneously, with backward elimination, to predict the difference in localization between the saline (similar) and sucrose (dissimilar) conditions for the oregano odor. The final model was significant, F(2, 14) = 4.21, p < .05, and accounted for 29% of the variance (adjusted R 2). Two predictors were retained in this model: the taste quality variable, which was not significant, and the hedonic variable, which was, p < .05. This finding is similar to that in Experiment 1, indicating that the more pleasant and foodlike oregano was judged to be, the smaller the difference in localization between the saline and sucrose conditions. The same approach was then used for the difference in localization for plum in sucrose minus plum in saline. In this case, the final model was also significant, F(1, 15) = 7.36, p < .025, and accounted for 29% of the variance (adjusted R 2). Here, judging plum to smell more intense was associated with a larger difference between the similar and dissimilar conditions.
Hedonic data
Finally, we examined whether the hedonic ratings for each taste and smell pair bore the same relationship across the four conditions, using the same approach as that described in Experiment 1 (with alpha set at .0125). The taste was judged as liked significantly less than the odor only for plum–saline (M = 2.8), t(16) = 6.95, p < .0125, and although plum–sucrose (M = 0.2) and oreg–saline (M = 1.2) were above zero and oregano–sucrose below (M = –1.3), they were not significantly so at this level of alpha.
Discussion
No effect of perceptual similarity on oral localization was obtained in Experiment 2, although all of the odor–taste pairs met the necessary conditions. In fact, the only suggestion of a difference obtained in this experiment concerned the oregano–sucrose condition, which received a lower (but not significantly so) localization score than did the oregano–saline combination. This difference seemed to parallel that for oolong tea in Experiment 1, in that both combinations were the only ones for which the odor was judged as less pleasant than the taste (albeit nonsignificantly for oregano–sucrose). Yet again, this would seem to suggest that it is the degree to which either the taste or the olfactory stimulus can command attention that may dictate localization. In Experiment 3, we attempted to test this hypothesis more directly.
Experiment 3
The data above suggest that manipulating the qualitative similarity of an odor and a taste has little impact on participants’ localization judgments. Instead, the data from the preceding experiments suggest that when an odor component becomes more attention demanding—notably, by being judged as less pleasant than the taste—this serves to reduce localization to the mouth. As was noted in the introduction, the affective response to a stimulus is only one feature that might demand attention, and others include stimulus intensity and pungency—namely, the degree to which trigeminal receptors are stimulated (a component of the somatosensory system; Cain & Murphy, 1980). Indeed, any factor that serves to draw attention to the nose should act to reduce oral localization, and any factor that serves to draw attention to the mouth should increase it. Experiment 3 set out to manipulate factors that should draw attention to the nose and factors that should draw attention to the mouth.
The attention-demanding nature of the stimuli in Experiment 3 was manipulated in several ways to maximize the likelihood of obtaining an effect—the parameters being largely unknown. First, participants completed a set of localization trials either with the odor alone or with the odor at a higher concentration mixed with a small quantity of glacial acetic acid. Most odorants stimulate both olfactory and nasal irritant receptors (the common chemical sense or trigeminal system; Laska, Distel, & Hudson, 1997), and especially so when the concentration of the odorant is increased (Cain & Murphy, 1980). Not only would this serve to increase the perceived intensity and pungency of the olfactory stimulus, it should also reduce its pleasantness and, thus, draw attention to the nose, thereby reducing localization to the mouth. Second, we varied the concentration and type of tastant used, such that two concentrations of sucrose were employed (weak and strong), along with one concentration of citric acid, sufficient to induce intensity ratings similar to those for the stronger sucrose solution. However, the citric acid sample would also likely be judged as less pleasant than the two sucrose samples and also as more pungent (irritating), since it too is a trigeminal irritant, as well as a tastant. On this basis, the greatest localization to the mouth should be observed with citric acid (disliked, intense and pungent) and then with the strong concentration of sucrose (intense), with the smallest effect for the weaker concentration of sucrose. Finally, these olfactory and taste manipulations should be independent, such that increasing attention to the olfactory/trigeminal channel should be equally effective at drawing attention away from any oral stimulus, and increasing attention to the oral stimulus should be equally effective at drawing attention away from the olfactory/trigeminal channel.
Method
Participants
Thirty participants (mean age = 25.5 years, SD = 4.5) took part in this experiment for a small cash payment.
Stimuli
The two target odorants were plum odor (0.18 g/L; Quest, Sydney) and plum odor (0.35 g/L) in 5% (w/v) glacial acetic acid (GAA; Sigma, Sydney). Each odor was presented in an aliquot of 20 ml in a transparent 250-ml screw-top jar. The three tastants were weak sucrose (30 g/L; Sigma-Aldrich, Sydney), strong sucrose (150 g/L), and strong citric acid (4 g/L; Sigma-Aldrich, Sydney), each presented as 10-ml samples in transparent disposable 20-ml cups. All the solutions were transparent and visually indistinguishable.
Procedure
As before, the basic task was to judge the source of an odor on each of 16 experimental trials, which were divided into two blocks of 8. Each block contained an identical set of trials that differed in only one respect: One block used plum odor, and the other used the plum–GAA mixture. Whether a participant got the plum block first or second was counterbalanced across participants. Each block contained 4 trials on which a jar containing an odorant was presented to the nose and 4 trials on which a jar containing water was presented to the nose. These were fully crossed with the mouth stimuli, each presented in a cup, which consisted of plain water, strong citric acid, weak sucrose, and strong sucrose. The order of presentation within a block was randomized separately for each block and for each participant. Since the order in which participants received each block had no significant effect on localization ratings, this variable is not reported in the analysis section.
Finally, participants undertook two further judgment tasks in counterbalanced order. On the odor judgment task, they evaluated the plum and plum–GAA odors. Each solution was sniffed 3 times, after which participants rated the stimulus using five 7-point category-rating scales: how intense, pungent, sweet, and sour it smelled (from 1 [not at all] to 7 [very]) and how much they liked or disliked it (from1 [dislike] to 4 [indifferent] to 7 [like]). On the taste judgment task, they evaluated sucrose and citric acid, swilling and spitting each solution, and then rating each taste for intensity, pungency, sweetness, sourness, and liking (same scales as above).
Results
Odor and taste ratings
Plum odor was judged as significantly less intense (M = 4.2), t(29) = 8.03, p < .001, and less pungent (M = 2.0), t(29) = 11.03, p < .001, than plum–GAA (Ms = 6.5 and 5.9, respectively). Plum odor was also judged to smell sweeter (M = 5.1), t(29) = 9.07, p < .001, and less sour (M = 1.9), t(29) = 5.47, p < .001, than plum–GAA (Ms = 1.9 and 4.5, respectively). Plum odor was liked more (M = 5.1), t(29) = 7.89, p < .001, than plum–GAA (M = 2.5). Thus, as we expected, plum–GAA should be a more salient stimulus—more intense, pungent, and unpleasant—than plum alone.
Each taste was compared on each rating, using a one-way ANOVA with follow-up Bonferroni-adjusted contrasts. Individual F tests are not reported, since all were significant, all Fs > 52.4, and only the probability value associated with each contrast is noted. Strong sucrose (M = 5.3) and citric acid (M = 5.9) did not significantly differ in intensity, but both were judged more intense than weak sucrose (M = 2.6), ps < .001. Strong sucrose was judged sweeter (M = 5.9), than weak sucrose (M = 4.0, p < .001), and both were judged as sweeter than citric acid (M = 1.5), ps < .001. Citric was judged sourer (M = 6.0), than both weak (M = 1.2) and strong (M = 1.2) sucrose, ps < .001, neither of which differed. Citric acid was judged as more pungent (M = 4.7) and was liked less (M = 2.3), than both weak (Ms = 1.2 and 4.8, respectively) and strong (Ms = 1.4 and 5.4, respectively) sucrose, ps < .001, neither of which differed. Thus, as was expected, the two strong tastes were equally intense but differed in pungency and affective valence, and for sucrose, the two concentrations differed in intensity.
Localization ratings
Figure 3 presents mean localization data for the two odors in each mouth condition. We started by examining whether localization for both odors combined was greater when the taste stimuli were present, relative to when water was in the mouth. The presence of a tastant generated significantly more localization toward the mouth (M = 2.7), or away from the nose, than did water in the mouth (M = 1.5), t(29) = 5.12, p < .001. In addition, the number of participants reporting localization to the mouth was examined (i.e., a score of 5 or more). When water was in the mouth, 2/30 participants reported oral localization on any trial, but when a taste was present, 20/30 reported localization on one or more trials, and this difference was significant [McNemar change test, χ 2(1) = 16.06, p < .001].
Mean localization ratings (and SEs) for each nose (stimulus presented for sniffing; GAA = glacial acetic acid) and mouth (stimulus presented for tasting) condition in Experiment 2
Localization data were analyzed using a two-way repeated measures ANOVA, with nose (plum vs. plum–GAA) and mouth (weak sucrose vs. strong sucrose vs. strong citric acid) as within-participants factors. The ANOVA revealed a main effect of nose, F(1, 29) = 8.79, MSE = 2.51, p < .01, partial η 2 = .23, with greater oral localization for plum than for plum–GAA (see Fig. 3), and a main effect of mouth, F(2, 58) = 9.71, MSE = 1.94, p < .001, partial η 2 = .25. Consistent with our hypothesis, there was a significant linear trend for mouth (high citric > high sucrose > low sucrose), F(1, 29) = 15.76, MSE = 2.37, p < .001, partial η 2 = .35, suggesting greater localization when citric acid was in the mouth, relative to strong and weak sucrose (there was no quadratic trend; F < 0.2). Finally, the interaction between nose and mouth was not significant, F < 0.1, indicating the independence of these two effects.
The number of participants reporting localization to the mouth was also examined (i.e., a score of 5 or more). For the three plum-with-taste trials, 17/30 participants reported an instance of oral localization (median = 1), while 9/30 did for plum–GAA (median = 0). Reports of oral localization were significantly greater in the plum condition (Wilcoxon test, Z = 2.12, p < .05). For the tastants, 15/30 participants reported an instance of oral localization on the two citric acid trials (median = 0.5), 12/30 for strong sucrose (median = 0), and 6/30 for weak sucrose (median = 0). Using Pages test for ordered alternatives, there was a significant difference between these three tastants in the a priori specified direction, Z = 2.07, p < .05. Using difference scores (i.e., tastant with plum – tastant with plum–GAA), we then tested for an interaction effect, but this was not significant (Friedman test). Thus, the pattern of results for just oral localization matched that using the whole localization score.
General discussion
Two principal findings emerged from this series of experiments. First, whether odors and tastes differ or not in perceptual similarity seems to have little bearing on participants’ propensity to localize an odor to the mouth. In Experiment 1, for vanilla–sucrose and vanilla–citric acid mixtures, and in Experiment 2, for plum–sucrose, plum–saline, oregano–sucrose, and oregano–saline mixtures, these all yielded similar localization scores, even though, for each set of pairs, there was a marked and highly significant disparity in perceived similarity between the taste and the smell. Perceptual similarity did, however, impact on one variable. Experiment 2 included odor intensity ratings, and these were significantly higher for the sweet-odor/sweet-taste condition, relative to the odor alone, suggesting an enhancement effect as observed before (e.g., Davidson et al., 1999; Von Sydow et al., 1974). The second outcome from these experiments concerned the relative salience of the elements in odor–taste mixtures and the impact this had on localization. Both Experiments 1 and 2 provided some evidence that where the odor was judged as more unpleasant than the taste, the degree of oral localization was reduced. This effect was significant and marked for the oolong tea–sucrose mixture in Experiment 1 and was evident, but not significant, for the oregano–sucrose mixture in Experiment 2. Experiment 3 directly manipulated the salience of oral and nasal factors that might be expected to draw attention to these respective locations. When the target odor here was presented at a higher concentration and with a nasal somatosensory irritant (GAA), this combination yielded less localization to the mouth than when just a lower concentration of the target odor was used. Manipulation of oral stimuli, by varying concentration of sucrose and inclusion of a higher concentration of citric acid, exerted the reverse effect. Here, the most orally attention-demanding stimulus—unpleasant, irritating, and intense citric acid—generated more localization than did intense sucrose, and this in turn generated more localization than did low intensity sucrose. These effects were also evident when a binary form of the data was used, which categorized participants as demonstrating or not demonstrating oral localization. Indeed, all the localization effects here could be demonstrated in this way.
Before turning to the implications of these results, it is important to consider potential shortcomings in the design and execution of these experiments. One issue in this regard is the reliability and validity of the localization procedure employed here. This rests upon a number of observations drawn from the experiments here and from other series either published (Stevenson et al., in press) or under submission (Stevenson, Tham, & Miller, 2010). In a previous series (Stevenson et al., in press), we found that participants’ localization ratings were reliable, r(15) = .81, at least over a short interval (15 min), suggesting some consistency in participants’ evaluations. The validity of this procedure is suggested both by its capacity to detect taste enhancement effects observed previously in flavor experiments (both here and in Stevenson et al., in press) and by viscosity flavor suppression effects (Stevenson et al., in press). An additional relevant finding is that in this study and all others, the water and no-oral-stimulus conditions yielded appropriate localization judgments—to the nose and external environment. Relatedly, and as we noted above, all our effects could be obtained using both mean localization scores and binary classification of participants into those reporting or not reporting oral localization (i.e., score greater than 5). This would seem to suggest that participants are able to experience some form of oral capture under conditions in which one would expect it to occur. A further related issue is whether, in fact, odor location is a binary state (mouth or nose) or a continuous one (i.e., mouth to nose). At present, there are no definitive data on this question; nonetheless, most previous investigators have treated it as a continuous variable (e.g., Small et al., 2005; von Bekesy, 1964), which is why we also adopted this approach. Finally, it may seem odd that our procedure did not include actual retronasal odor presentations. However, there are several reasons for this. First, this would have involved a different administration strategy for ortho- versus retronasal odor presentations, since retronasal presentation would require a reversal of airflow (exhalation), relative to orthonasal presentation (inhalation). Second, since direction of airflow has been noted before as a potential basis for localization (Hummel et al., 2006; Small et al., 2005), this would make it hard to isolate the impact of events in the mouth in this process. Third, we felt that delivering odors othonasally would provide the most emphatic demonstration of oral capture, simply because it does not involve retronasal presentation.
In the introduction, we canvassed two possible outcomes for the experiments here. One suggested that it would be harder to voluntarily attend to the odor component in perceptually similar combinations, generating greater oral localization relative to perceptually dissimilar pairs. The other suggested that it was the capacity of the stimulus elements in the flavor to command attention that was important and that tastes were likely to be good candidates in this regard, since they may be valanced, intense, and sometimes pungent stimuli. The data from the experiments here are most consistent with this second possibility. In Experiment 1—and to some extent in Experiment 2—oral localization was reduced only under conditions in which the odor was substantially more negatively valanced than the accompanying taste, arguably then providing the conditions for the odor to command attention. If oral localization, then, is affected by the capacity of a particular cue to command attention, we might expect any variable that acts to make a particular cue more attention demanding—affect being one, intensity and pungency others—to also affect localization. In this regard, Experiment 3 demonstrated that altering the salience of the olfactory channel (or more strictly, the olfactory/nasal somatosensory channel) reduced oral localization, while increasing the salience of the oral-based stimuli had the reverse effect. These observations are consistent with the idea that it is the capacity of individual flavor elements to command attention, which will dictate the degree to which an odor is localized to the mouth or nose.
As we noted in the introduction, the mechanisms that contribute to localization of an odor to the mouth are likely to be related to the mechanisms responsible for flavor binding. Indeed, these may be one and the same thing. If flavor binding were to rely upon the ability of particular features to command attention, this might have two implications. First, it might make it hard to voluntarily shift attention away from the most salient feature (element). Evidence consistent with this has been observed, in that attempts to voluntarily attend to the olfactory component of a flavor confers no benefit on detecting that element in an odor–taste mixture (Ashkenazi & Marks, 2004). Second, there is an arguably closer meshing between the basic function of flavor perception (i.e., to secure safe nutritious food) and the exogenous attentional account (i.e., what commands attention dictates localization and, perhaps, flavor binding) than with the endogenous attentional account (i.e., what can be attended dictates localization and, perhaps, flavor binding). This is suggested by a functional analysis of flavor binding. The brain needs to learn about flavor so that when cues that compose that flavor are encountered again, the flavor can be recalled (this is presumably why foods that contain sugars [energy] come to smell sweet/pleasant and why foods that have made us sick [poison] smell foul). This need to learn may dictate the general tendency to integrate taste and smell that we noted in the introduction. The specific integration effect, which manifests as perceptual similarity—or congruency, in the case of familiar combinations—may then be a side effect (i.e., a consequence) of this need to learn with little functional relevance to events in the mouth. A functional perspective would also suggest that an unduly intense, pungent, or novel feature should be found unpleasant, and this should also be attention demanding. This is because ingesting food with unduly intense, pungent, or novel (i.e., unpleasant) features may be fatal, signaling the possible presence of poisons or pathogens (e.g., degree of bitterness is strongly correlated with LD50; T. R. Scott & Mark, 1987). Salient elements of a flavor may then command attention, whether we choose to attend to them or not, precisely to avoid such consequences, and this may be why our voluntary capacity to detect odors in oral taste mixtures has little functional value and, thus, a limited role in odor localization and flavor binding.
References
Ashkenazi, A., & Marks, L. E. (2004). Effect of endogenous attention on detection of weak gustatory and olfactory flavors. Perception & Psychophysics, 66, 596–608.
Cain, W. S., & Murphy, C. (1980). Interaction between chemoreceptive modalities of odour and irritation. Nature, 284, 255–257.
Clark, C. C., & Lawless, H. T. (1994). Limiting response alternatives in time-intensity scaling: An examination of the halo-dumping effect. Chemical Senses, 19, 583–594.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus driven attention in the brain. Nature Reviews. Neuroscience, 3, 201–215.
Dalton, P., Doolittle, N., Nagata, H., & Breslin, P. A. S. (2000). The merging of the senses: Integration of subthreshold taste and smell. Nature Neuroscience, 3, 431–432.
Davidson, J. M., Linforth, R. S. T., Hollowood, T. A., & Taylor, A. J. (1999). Effect of sucrose on the perceived flavor intensity of chewing gum. Journal of Agricultural and Food Chemistry, 47, 4336–4340.
Deems, D. A., Doty, R. L., Settle, R. G., Moore-Gillon, V., Shaman, R., & Mester, A. F. (1991). Smell and taste disorders: A study of 750 patients from the University of Pennsylvania smell and taste center. Archives of Otolaryngology - Head & Neck Surgery, 117, 519–528.
Frank, R. A., & Byram, J. (1988). Taste–smell interactions are tastant and odorant dependent. Chemical Senses, 13, 445–455.
Frasnelli, J., Heilmann, S., & Hummel, T. (2004). Responsiveness of human nasal mucosa to trigeminal stimuli depends on the site of stimulation. Neuroscience Letters, 362, 65–69.
Green, B. G. (2002). Studying taste as a cutaneous sense. Food Quality and Preference, 14, 99–109.
Hummel, T., Heilmann, S., Landis, B. N., Reden, B. N., Frasnelli, J., Small, D. M., & Gerber, J. (2006). Perceptual differences between chemical stimuli presented through the orth- or retronasal route. Flavour and Fragrance Journal, 21, 42–47.
Laska, M., Distel, H., & Hudson, R. (1997). Trigeminal perception of odourant quality in congenital anosmic subjects. Chemical Senses, 22, 447–456.
Murphy, C., & Cain, W. S. (1980). Taste and olfaction: Independence vs interaction. Physiology & Behavior, 24, 601–605.
Posner, M. I. (1980). Orienting of attention: The VIIth Sir Frederic Bartlett lecture. The Quarterly Journal of Experimental Psychology, 32A, 3–25.
Rozin, P. (1982). “Taste–smell confusions” and the duality of the olfactory sense. Perception & Psychophysics, 31, 397–401.
Rozin, P., & Royzman, E. B. (2001). Negativity bias, negativity dominance and contagion. Personality and Social Psychology Review, 5, 296–320.
Scott, J. W., Acevedo, H. P., Sherrill, L., & Phan, M. (2007). Responses of the rat olfactory epithelium to retronasal air flow. Journal of Neurophysiology, 97, 1941–1950.
Scott, T. R., & Mark, G. P. (1987). The taste system encodes stimulus toxicity. Brain Research, 414, 197–203.
Small, D. M., Gerber, J. C., Mak, Y. E., & Hummel, T. (2005). Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron, 47, 593–605.
Stevenson, R. J., & Oaten, M. (2010). Sweet odours and sweet tastes are conflated in memory. Acta Psychologica, 134, 105–109.
Stevenson, R. J., Oaten, M. J., & Mahmut, K. M. (in press). The role of taste and oral somatosensation in olfactory localisation. Quarterly Journal of Experimental Psychology.
Stevenson, R. J., Prescott, J., & Boakes, R. A. (1999). Confusing taste and smells: How odours can influence the perception of sweet and sour tastes. Chemical Senses, 24, 627–635.
Stevenson, R. J., Tham, W. P., & Miller, L. A. (2010). Impaired olfactory attention in participants with mediodorsal thalamic lesions: Effects on oral capture, odor-induced taste synesthesia and flavor binding. Manuscript submitted for publication.
Valentin, D., Chrea, C., & Nguyen, D. H. (2006). Taste-odour interactions in sweet taste perception. In W. Spillane (Ed.), Optimising sweet taste in foods (pp. 66–84). Cambridge: Woodhead.
Van der Klaauw, N. J., & Frank, R. A. (1996). Scaling component intensities of complex stimuli: The influence of response alternatives. Environment International, 22, 21–31.
Von Bekesy, G. (1964). Olfactory analogue to directional hearing. Journal of Applied Physiology, 19, 363–373.
Von Sydow, E., Moskowitz, H., Jacobs, H., & Meiselman, H. (1974). Odor–taste interaction in fruit juices. Lebensmittel-Wissenschaft und Technologie, 7, 18–24.
White, T. L., & Prescott, J. (2007). Chemosensory cross-modal stroop effects: Congruent odors facilitate taste identification. Chemical Senses, 32, 337–341.
Acknowledgments
We thank Samantha Baggot and Tracey Shaw for their assistance with these experiments and the Australian Research Council for their continued support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stevenson, R.J., Mahmut, M.K. & Oaten, M.J. The role of attention in the localization of odors to the mouth. Atten Percept Psychophys 73, 247–258 (2011). https://doi.org/10.3758/s13414-010-0013-6
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-010-0013-6