Abstract
Alcohol intoxication is implicated in approximately half of all violent crimes. Over the past several decades, numerous theories have been proposed to account for the influence of alcohol on aggression. Nearly all of these theories imply that altered functioning in the prefrontal cortex is a proximal cause. In the present functional magnetic resonance imaging (fMRI) experiment, 50 healthy young men consumed either a low dose of alcohol or a placebo and completed an aggression paradigm against provocative and nonprovocative opponents. Provocation did not affect neural responses. However, relative to sober participants, during acts of aggression, intoxicated participants showed decreased activity in the prefrontal cortex, caudate, and ventral striatum, but heightened activation in the hippocampus. Among intoxicated participants, but not among sober participants, aggressive behavior was positively correlated with activation in the medial and dorsolateral prefrontal cortex. These results support theories that posit a role for prefrontal cortical dysfunction as an important factor in intoxicated aggression.
Similar content being viewed by others
Alcohol is the most common psychotropic contributor to aggressive behavior. In many parts of the world, acute alcohol consumption is implicated in approximately 35% to 60% of violent crimes. Population-based research shows that alcohol is associated with many forms of violence, including homicide, physical assault, sexual assault, intimate partner violence, and child abuse (Foran & O’Leary, 2008; Hoaken & Stewart, 2003; Murdoch & Ross, 1990; Quigley & Leonard, 2000; Room & Rossow, 2001; U.S. Department of Justice, 1998; WHO, 2006). Meta-analyses of placebo-controlled experiments consistently reveal small to moderate effects of acute alcohol consumption on aggressive behavior in laboratory studies (Bushman, 1993; Bushman & Cooper, 1990; Hull & Bond, 1986; Ito, Miller, & Pollock, 1996; Steele & Southwick, 1985).
By itself, alcohol consumption is typically considered insufficient to spontaneously elicit aggressive behavior (Graham, 1980). However, when combined with hostile situations or dispositional aggressiveness, alcohol can promote aggressive behavior. Finkel and colleagues’ I3 theory (pronounced “I-cubed theory”) provides a conceptual basis for understanding how alcohol can impact aggressive behavior (Denson, DeWall, & Finkel, 2012; Finkel & Hall, 2017). According to I3 theory, three processes cause aggression: instigation, impellance, and inhibition. Instigating factors are those that arouse aggressive tendencies in most people, such as provocation or social rejection. Impelling factors are situational or dispositional characteristics that enhance the severity of aggression (e.g., a long history of prior conflict). Inhibition processes refer to factors that can prevent aggression (e.g., high self-control). According to I3 theory, alcohol reduces self-control and can thereby promote aggression when instigating and/or impelling factors are present (Graham, 1980; Heinz, Beck, Meyer-Lindenberg, Sterzer, & Heinz, 2011). In accordance with I3 theory, there is much evidence that people who possess an alcohol use disorder or strong impelling dispositions toward violence commit a disproportionate amount of alcohol-related violence (Fals-Stewart, Leonard, & Birchler, 2005; Parrott & Zeichner, 2002; Swanson, Holzer, Ganju, & Jono, 1990; for a similar effect in mice, see Miczek, Barros, Sakoda, & Weerts, 1998). In the present research, we focused on instigation (i.e., provocation) and disinhibition (i.e., alcohol intoxication) in healthy young men in order to provide a basis for future work on the neural correlates of alcohol-related aggression in violence-prone individuals.
Although little neuroimaging research has examined intoxicated aggression, there is a growing body of research that is discovering the neural mechanisms of sober aggression. Most of these studies have used the Taylor (1967) Aggression Paradigm (TAP), which has been modified for fMRI research (e.g., Krämer, Jansma, Tempelmann, & Münte, 2007; Lotze, Veit, Anders, & Birbaumer, 2007). During the TAP’s decision phase, participants behave aggressively by selecting noxious stimuli to be sent to a provoking or nonprovoking opponent. During these acts of aggression, participants typically show increased blood-oxygen-level-dependent (BOLD) responses in prefrontal regions, subcortical limbic structures, and regions associated with reward processing (Krämer et al., 2007; Krämer, Riba, Richter, & Münte, 2011; Lotze et al., 2007). Specifically, studies found that the TAP activated the dorsolateral prefrontal cortex (PFC), ventrolateral PFC, medial PFC, anterior cingulate, amygdala, putamen, caudate, thalamus, insula, ventral striatum, and hippocampus (Chester & DeWall, 2016; Emmerling et al., 2016; Krämer et al., 2007; Lotze et al., 2007). Thus, behaving aggressively activates neural responses associated with negative affect, arousal, cognitive-behavioral control, mentalizing, and reward.
The specific regions responsible for producing aggressive behavior remain under investigation. Some studies have shown positive associations between reward regions and aggression. Activation in the caudate (Beyer, Münte, Erdmann, & Krämer, 2014) and nucleus accumbens (Chester & DeWall, 2016) during the TAP positively correlated with aggressive behavior. The role of the PFC remains less clear. Lotze et al. (2007) found that activation in the medial PFC positively correlated with the extent of pain selected for the opponent in the TAP, possibly due to negative mentalizing about the opponent. Similarly, Chester et al. (2013) found that dorsal anterior cingulate activation in response to social exclusion positively predicted aggression, but only among participants low in executive functioning. However, another study found inverse relationships between aggressive behavior and activation in the dorsolateral PFC, amygdala, and hippocampus (Achterberg, van Duijvenvoorde, Bakermans-Kranenburg, & Crone, 2016). Manipulations of frontal hemispheric dominance via transcranial direct current stimulation effectively reduced aggression when applied to the right ventrolateral PFC (Riva et al., 2015) but did not reduce reactive aggression when applied to the right dorsolateral PFC (Dambacher et al., 2015b). Thus, in sober participants, the lateral PFC may inhibit aggressiveness, whereas the medial PFC may promote aggressiveness. More research is needed on the neural mechanisms responsible for sober and intoxicated aggression.
Acute alcohol consumption increases serotonin and dopamine release while simultaneously exerting inhibitory action in the cortex through gamma-aminobutyric acid release. The putative proximal mechanism through which acute alcohol consumption enhances aggression is reduced activity in the PFC (Heinz et al., 2011). Heinz et al. (2011) reviewed psychological and biological theories of alcohol and aggression. Nearly all of the theories implicated decreased activation in the PFC in some manner. Furthermore, in hostile situations, alcohol-induced reduction in PFC activity is thought to weaken the regulation of activity in the ventral striatum and amygdala (Gan, Sterzer, Marxen, Zimmermann, & Smolka, 2015; Heinz et al., 2011). Indeed, in one study of heavy social drinkers, acute alcohol intoxication weakened coupling between the amygdala and orbitofrontal cortex when viewing emotional faces (Gorka, Fitzgerald, King, & Phan, 2013).
The alcohol-induced disruption to the PFC is evidenced by relatively poor performance on numerous tests of executive functions. Indeed, when participants consumed alcohol relative to placebo, they showed impairments in cognitive abilities mediated by the PFC, such as attentional control, planning, set switching, response inhibition, and information processing (Easdon & Vogel-Sprott, 2000; Finn, Justus, Mazas, & Steinmetz, 1999; Guillot, Fanning, Bullock, McCloskey, & Berman, 2010; Lyvers & Maltzman, 1991; Peterson, Rothfleisch, Zelazo, & Pihl, 1990; Schreckenberger et al., 2004). Furthermore, fMRI studies have observed alcohol-induced reductions in PFC activity during inhibitory control tasks (Anderson et al., 2011; Gan et al., 2014; Kareken et al., 2010). In turn, these executive functioning deficits are thought to increase the risk for reactive aggression and other impulsive behaviors (Giancola, 2000).
One recent fMRI study compared neural responses to provocation in alcohol-dependent men to neural responses in control participants (Kose et al., 2015). Specifically, participants played an aggression paradigm in which participants were provoked by having money stolen from them (Cherek, 1981). When provoked, compared to control participants, alcohol-dependent participants showed reduced responses in the PFC, thalamus, and hippocampus. The relationship between neural responses to provocation and aggressive behavior was not moderated by participant group. However, when both groups were analyzed together, the authors found negative relationships between aggression and BOLD responses in the PFC (including the orbitofrontal cortex), thalamus, middle temporal gyrus, and caudate. These findings point to the notion that the PFC, limbic system, and reward processing regions are critically involved in alcohol-induced aggression. However, we know almost nothing about alcohol-related brain activity during acts of aggression. Placebo-controlled neuroimaging experiments that manipulate alcohol intoxication and measure brain activity during aggressive behavior are needed to test the hypothesis that disruption in the PFC influences alcohol-related aggression.
To our knowledge, there has been only one placebo-controlled neuroimaging study of alcohol and aggression to date (Gan et al., 2015). In that notable experiment, 35 healthy young men and women completed the Taylor Aggression Paradigm in the scanner once while intoxicated and once after consuming a placebo. Alcohol decreased BOLD responses in the right PFC (i.e., middle frontal and inferior frontal gyri), hippocampus, thalamus, caudate, and putamen. Moreover, activity in the amygdala and ventral striatum was not affected by alcohol but was positively correlated with alcohol-induced aggression against the provoking opponent. However, their study differed from the present research in that they examined BOLD responses when participants found out they were going to play against a provoking or nonprovoking participant. Thus, they examined anticipatory neural responses, whereas our experiment examined neural activity during aggressive decision-making and behavior.
The present study
To further identify the neural mechanisms responsible for intoxicated aggression, 50 healthy young men consumed either alcohol or placebo. They then completed the Taylor Aggression Paradigm during scanning, ostensibly against two opponents. We examined brain activation when participants made the decision to aggress and acted on that decision (Krämer et al., 2007). We considered two distinct possible outcomes. First, alcohol could globally reduce PFC, limbic, and reward activation, which could then correlate with aggressive behavior. Second, alcohol may moderate the relationship between PFC or subcortical activity and aggressive behavior. That is, these regions may support different behaviors (i.e., peace vs. aggression) depending on whether participants are sober or intoxicated.
Method
Participants and design
Participants were recruited via an advertisement on a job-search website or local community entertainment newspaper that offered payment of AUD$75 in exchange for research participation. There was no mention of alcohol in the advertisements in order to avoid recruiting a biased sample. During an initial phone screening, those who reported poor physical or mental health, medication that contraindicated alcohol, previous adverse reactions to alcohol, or did not drink 3 or more times per month were excluded. Participants who scored ˃15Footnote 1 on the Alcohol Use Disorders Identification Test (AUDIT) were excluded. Remaining eligible participants were invited to attend an in-person interview that included the administration of the Mini-International Neuropsychological Inventory (MINI) to screen for participants who had suffered, or were currently suffering, from an Axis I disorder. Participants were asked to abstain from eating, consuming caffeine, or exercising 2 hours prior to the experiment and drinking alcohol 24 hours prior to the experiment. Further exclusion criteria included endocrine, metabolic or immune disorders, and cancer, smoking, and regular recreational drug use. We set these exclusion criteria because we also assessed salivary hormones, which were not analyzed. All scanning took place in the afternoon to early evening.
The 60 participants identified as eligible were then invited to continue their participation. Sample size was set at 60 due to limited funding. Participants were informed they might be asked to drink an alcoholic beverage and were randomly assigned to either the alcohol or placebo condition. Data from 10 participants were excluded from analysis: six for technical errors during the scanning session, one for excessive movement that could not be motion corrected, one due to an abnormal structural scan determined by a radiologist, and two who did not believe they were playing with real participants. Therefore, the final sample consisted of 50 participants (Mage = 23.00 years, SD = 3.28, range: 18–30 years) who were predominantly Caucasian (68%) or Asian (20%). There were 23 participants in the alcohol condition and 27 in the placebo condition.
Materials and procedure
During an initial session in our laboratory, participants were screened for right-handedness and MRI safety and completed the Aggression Questionnaire (Buss & Perry, 1992) as well as other questionnaires we did not analyze. The Aggression Questionnaire consists of four subscales: Anger, Hostility, Verbal Aggression, and Physical Aggression (1 = extremely uncharacteristic of me to 5 = extremely characteristic of me). The Physical Aggression subscale was the only subscale significantly correlated with aggressive behavior during the scanning task, r(48) = .28, p = .045. There were no group differences in any of the subscales, F< 1 (see Table 1). At the completion of these measures, participants weight and body fat percentage were measured using an electronic scale in order to establish the amount of alcohol (or placebo) to be administered in the scanning session based on lean body fat percentage.
Alcohol manipulation
During the second session, which took place at Neuroscience Research Australia, participants took part in a practice round of the aggression task. The practice was performed prior to drink consumption. We also obtained a baseline breath alcohol concentration (BrAC) with a calibrated Alcolizer LE (Alcolizer Technology, Australia), which is used by police in New South Wales, Australia. Participants consumed two alcoholic beverages (the calculated amount from Session 1 was divided equally between the two drinks) or placebo beverages, depending on their allocated condition. We administered 0.72 grams of alcohol per kilogram of lean body mass (i.e., 2.42 ml of 37.5% alcohol by volume [ABV] vodka per kilogram of lean body mass). This formula was used in prior research to achieve our target BrAC level of .05%, which is the point at which it is illegal to drive in Australia (Schofield, Unkelbach, & Denson, 2017). The alcoholic beverage was made up of 37.5% ABV vodka, sugar-free lemonade, and diet tonic water (1:1:2). Drinks were mixed in front of the participants. The placebo condition was identical, except that the alcohol was substituted with additional decarbonated tonic water poured from a vodka bottle. A small amount of vodka was also discretely smeared on the rim of the plastic cup to provide an odor of alcohol and further convince the participant. Thus, all participants were led to believe they were consuming alcohol and were not told which condition they were in.
Prescan measures
As a manipulation check, an additional item assessed subjective intoxication (i.e., intoxicated) on an 11-point scale (0 = not at all, 10 = very much). Upon the completion of the task practice and after finishing the beverages, participants were escorted to the MRI reception. The second BrAC measurement was then recorded. Participants rinsed their mouth with water before this measurement was taken to avoid inflated readings.
Aggression task
Participants were told that they would be competing in a competitive reaction-time task, with auditory distractions with two other male participants, while in the scanner. They were also told that they would be the only one in the scanner and the other participants were completing the task at the neighboring University of New South Wales (UNSW). In reality, the participant was competing against two computer opponents (Opponent 1 and Opponent 2). The task was a modified version of the TAP adapted from Krämer et al. (2007), which allows participants to send a retaliatory noise blasts to their opponent. The task has been widely used in nearly 50 years of laboratory experiments and more recently in fMRI research (Chester & DeWall, 2016; Dambacher et al., 2015a; Gan et al., 2015; Krämer et al., 2011).
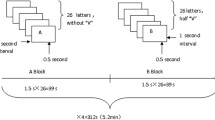
The task was two runs of 20 trials each (10 trials against Opponent 1 and 10 against Opponent 2 per run; see Fig. 1). Participants were asked to press a button on the button box as fast as possible when a colored square appeared on-screen. The task also required participants to make a selection of one of four noise intensities at the beginning of each trial (1 being the lowest intensity and 4 the highest) to be delivered to their opponent for that trial if the participant won. Likewise, the participant would hear the noise blast selected by the opponent if the participant lost. Wins and losses were randomized across trials, but if participants did not respond within the 9,000-ms window, they automatically lost. Opponent 1 was the low provocation opponent (i.e., selected noise blast intensities of 1 and 2) and Opponent 2 was the high provocation opponent (i.e., selected noise blast intensities of 3 and 4). To make sure participants understood the intent of the opponent, the opponent’s selection was revealed to the participant regardless of who won the trial. Aggressive behavior was operationalized by the mean noise intensity selection across the 20 trials for each opponent. Reliability was good for the low provocation (α = .94) and high provocation opponents (α = .91). There was no option to select the duration of the blast. Mean intensity was a priori coding method, and no alternate methods were considered. We calculated separate aggression scores designated for the low provocation and high provocation opponents. Because aggression occurs during the decision phase, we focused exclusively on this phase of the experiment (as in Chester & DeWall, 2016; Dambacher et al., 2015b; Emmerling et al., 2016). Each run was 1,020 seconds long (i.e., 17 minutes).
Manipulation checks and debrief
Immediately after the scan, participants completed a third BrAC. Participants then completed items to assess perceptions of each opponent (five items; e.g., who was meaner, prefer to meet, nicer, get along better with, friendlier?) and whether they thought they had consumed any alcohol (two items; e.g., how many standard drinks do you think you consumed?). Finally, participants were probed for suspicion, thanked, debriefed, and compensated. Participants were not permitted to leave the session until their BrAC was below 0.05 and were compensated for their time if the session was extended. All participants were also supplied with a prepaid public transport ticket home.
Image acquisition
Participants viewed the tasks through mirrors, which were presented on a high-resolution monitor placed at the end of a Philips Achieva X-Series 3-Tesla whole-body scanner with a 32-channel head coil and parallel imaging system. Padded foam head constraints controlled movement. We acquired a T1 anatomical 3-D structural data set (180 slices, FOV = 256 mm, voxel size = 1 × 1 × 1 mm). For functional imaging, a whole-brain EPI pulse sequence with sagittal slices and 2.5 SENSE acceleration was employed (59 slices, slice thickness = 3 mm, voxel size = 2.26 × 2.26 × 2.80 mm, FOV = 253 mm, TE = 50 ms, TR = 3,000 ms, 90° flip angle). The first four volumes were discarded.
Preprocessing
The sagittal EPI slices imaged substantial amounts of nonbrain tissue that could interfere with motion correction. Accordingly as a first step, BET from the FSL package (Smith et al., 2004) was used to remove all nonbrain components in the EPI images. After this step, the data were imported to BrainVoyager QX with which all subsequent preprocessing was performed. Functional images were slice scan time corrected, 3-D motion corrected, and spatially smoothed with a 4.52-mm Gaussian filter. Functional images were subsequently manually linearly coregistered with the structural images and normalized via Talairach transformation (Talairach & Tournoux, 1988). For the functional scans, we modeled all phases at the first level, adjusted for the hemodynamic response function. All phases except the decision phase were modeled as variables of no interest. Thus, BOLD during the decision phase was observed relative to all other phases.
Regions-of-interest (ROI) definitions
We investigated six ROIs: PFC, hippocampus, thalamus, caudate, ventral striatum, and amygdala (see Fig. 2). Because theories of alcohol-induced aggression posit alterations in PFC activity (Heinz et al., 2011), we created one ROI for the PFC, which subsumed the medial PFC, lateral PFC, and anterior cingulate. The PFC midline extended ±10 mm. Similarly, because the amygdala and ventral striatum correlated with aggressive behavior in prior research on alcohol-related aggression (Gan et al., 2015), we included these two ROIs as well. Finally, we included the hippocampus, thalamus, and caudate because they are activated in fMRI studies of aggression (Achterberg et al., 2016; Beyer et al., 2014; Chester & DeWall, 2016; Emmerling et al., 2016; Krämer et al., 2007; Lotze et al., 2007). For the amygdala, hippocampus, caudate, and thalamus, we used the ROIs included in the BrainVoyager QX software. For the ventral striatum, we created a 10-mm cube centered at x = ±16, y = 5, z = −5 as determined by the Brede Toolbox web application (http://neuro.imm.dtu.dk/services/brededatabase/WOROI_135.html). The PFC ROI was defined anatomically on an a priori basis in Talairach space on an average of all 50 participants’ Talairach-transformed structural images.
Statistical analyses
Data were analyzed with BrainVoyager QX, SPSS v23, and R (R Core Team, 2016). We first used ANOVAs to test the efficacy of the alcohol manipulation on BrAC and subjective intoxication. For BOLD responses, we conducted random effects GLM analyses. Our analytic strategy utilized three random effects contrasts: (1) alcohol main effect (alcohol group > placebo group); (2) provocation main effect (high provocation opponent > low provocation opponent); and (3) the Alcohol × Provocation interaction. We conducted six sets of analyses, one for each ROI mask. We used an AR(2) correction for serial correlations. The ROI analyses used the false discovery rate correction, q(FDR) < .05.
We next examined the neural correlates of aggressive behavior. We conducted an image-based correlation analysis in BrainVoyager QX, in which noise-blast intensity averaged across all trials and both opponents was used to predict activation within each of the masks. For the PFC mask, we created a second mask of all of the activated clusters greater than 100 voxels. We conducted separate analyses for the alcohol and placebo group and then tested for significant differences by subtracting the placebo group correlation map from the alcohol group’s map. Type I error was controlled at q(FDR) < .05. In the presences of significant group differences, to obtain estimates for testing the Alcohol × BOLD interactions and visualizing the correlations between BOLD and aggressive behavior in R, we exported the average beta estimates for the decision phase for each participant. We used these beta estimates as predictors in an ordinary least squares regression analysis.
Results
Manipulation checks and mood
All participants had a BrAC of .000 at baseline. Prior to entering the scanner, participants in the alcohol condition had a mean BrAC of .048 (SD = .018) and at the end of the experiment had a mean BrAC of .044 (SD = 0.15). This change in BrAC was not significant, t(21) = 1.34, p = .19. A 2 (condition) × 3 (time) mixed ANOVA on subjective feelings of intoxication revealed a significant Condition × Time interaction, F(2, 96) = 15.52, p = .007, ηp2 = .099. At baseline, participants in both the alcohol and placebo conditions reported equivalently low levels of intoxication, F < 1. Prior to entering the scanner and at the end of the study, participants in the alcohol condition reported feeling more intoxicated than participants in the placebo condition, F(1, 48) = 8.30, p = .006, ηp2 = .147, and F(1, 48) = 6.18, p = .016, ηp2 = .114, respectively. However, more importantly, both groups reported large increases in subjective intoxication from baseline: alcohol condition, F(1, 22) = 188.14, p < .0001, ηp2 = .895; placebo condition, F(1, 26) = 64.84, p < .0001, ηp2 = .714 (see Fig. 3). Participants in the alcohol condition thought they consumed 3.65 standard drinks on average (SD = 1.07), whereas participants in the placebo condition thought they consumed 2.86 standard drinks (SD = 1.37). Although this difference was significant, F(1, 46) = 4.94, p = .031, ηp2 = .097, participants in both groups reported consuming significantly more standard drinks than zero, talcohol(22) = 16.36, p < .0001; tplacebo(24) = 10.47, p < .0001. Together, these data suggest effective alcohol and placebo procedures.
Exact binomial tests revealed that the majority of participants reported that the low provocation opponent was friendlier (70%, p = .007) and nicer (68%, p = .015) than the high provocation opponent. Conversely, the high provocation opponent was rated as meaner (68%, p = .015) than the low provocation opponent. There were no differences in perceptions of whether the opponents were easier to get along with (58%, p = .32) and whether participants would prefer to meet them (60%, p = .20). Overall, these data suggest that participants were sensitive to the degree of provocation from each opponent. None of these judgments varied as a function of beverage condition.
Aggressive behavior
We conducted a 2 (condition: alcohol vs. placebo) × 2 (provocation: high vs. low) linear mixed-effects model on aggressive behavior (see Fig. 4). We specified participant as a random factor and condition and opponent as fixed factors. This analysis revealed a main effect of opponent such that participants were more aggressive toward the high provocation opponent (M = 2.64, SD = 0.76) than the low provocation opponent (M = 2.39, SD = 0.89), t(1876) = 3.52, p = .0004. The Condition × Provocation interaction approached significance, t(1876) = −1.82, p = .068, but none of the post hoc comparisons showed significant effects of alcohol as a function of provocation.
Aggression-related BOLD responses
Table 2 and Fig. 5 summarize the random effects GLM contrasts for each of the six ROIs controlling for multiple comparisons, q(FDR) < .05. There were several main effects of condition. Within the PFC mask, the main effect of alcohol revealed six significant clusters. These regions included the left middle and left inferior frontal gyri as well as the bilateral medial frontal gyrus. In all six PFC clusters, participants in the alcohol group showed reduced activation relative to participants in the placebo group. There was also a main effect of condition within the caudate and ventral striatum masks. BOLD responses in the caudate were lower in the alcohol group than in the placebo group. In contrast to the deactivations in the PFC, caudate, and ventral striatum, participants in the alcohol group showed greater bilateral activation in the hippocampus than participants in the placebo group. There were no other alcohol main effects for the remaining ROI masks and no main effects of provocation or Alcohol × Provocation interactions for any of the ROIs. In sum, BOLD responses were lowered by alcohol in the PFC, caudate, and ventral striatum yet heightened in the hippocampus. For uncorrected analyses and for correlations between BrAC and BOLD within the alcohol group, please see the supplementary materials.
Main effect of alcohol on aggression-related neural activity. Compared to the placebo group, participants who consumed alcohol showed less activity in the prefrontal cortex, caudate, and ventral striatum, but increased activity in the hippocampus, q(FDR) < .05. Values on bar charts are means. Error bars are ±1 SEM. (Color figure online)
The relationships between aggression-related BOLD responses and aggressive behavior
Aggressive behavior correlated with BOLD responses in the prefrontal cortex. The top portion of Fig. 6 displays the prefrontal clusters that were correlated with aggressive behavior in the scanner. Aggressive behavior was significantly positively correlated with BOLD during the decision phase but only in the medial and dorsolateral PFC among participants who consumed alcohol. A complementary multiple regression analysis of moderation (Aiken, West, & Reno, 1991), with the exported beta estimates from the prefrontal cortex, confirmed a significant Condition × PFC interaction, β = −0.69, t(46) = −2.57, p = .013, model R2 = .16, F(3, 46) = 2.85, p = .048. As shown in the scatterplot (see Fig. 6), BOLD responses were positively correlated with aggressive behavior among intoxicated participants, β = 0.69, t(21) = 4.40, p = .0003, R2 = .48, F(1, 21) = 19.32, p = .0003, but not among sober participants, β = −0.06, t(25) = −0.31 p = .76, R2 = .004, F(1, 25) = 0.10, p = .76. There were no correlations between aggressive behavior and BOLD responses within any of the remaining ROI masks, q(FDR) < .05.
Correlations between BOLD signal during the decision phase and aggressive behavior in the scanner. Medial and dorsolateral PFC activity positively correlated with aggressive behavior, but only for participants who consumed alcohol. The two panels on the left show significant correlations in the alcohol group, whereas the middle panels show no correlations between PFC activity and aggressive behavior in the placebo group. The right two panels display the differences in correlation strength by subtracting the placebo group’s correlation map from the alcohol group’s correlation map. Familywise error rate (FWE) was controlled with q(FDR) < .05. Shaded regions represent 95% CIs. (Color figure online)
Discussion
Although most theories of alcohol-related aggression implicate altered functioning in the PFC, substantial neuroimaging evidence is lacking. The present research tested this assumption, and the data supported the notion that altered PFC function contributes to intoxicated aggression. Relative to sober participants, we found decreases in PFC activation among intoxicated participants. This finding supports models of alcohol-related aggression that implicate PFC dysregulation. Furthermore, we found that BOLD responses in the dorsomedial and dorsolateral PFC positively correlated with aggressive behavior, but only among intoxicated participants. These findings suggest that when intoxicated, the PFC becomes dysregulated relative to sobriety, but that the activity that is present may facilitate intoxicated aggression.
In addition to PFC dysfunction, neurobiologically informed models of intoxicated aggression posit a role for altered functioning among subcortical limbic and reward structures (Heinz et al., 2011). Gan et al. (2015) found that alcohol lowered medial PFC and dorsal anterior cingulate activation among intoxicated participants. Our study replicated these findings. Gan et al. (2015) also found that activation in the ventral striatum and amygdala positively predicted intoxicated aggression. We did not find those relationships in our data, but we did find alcohol-induced reductions in the ventral striatum and caudate.Footnote 2 Differences in study design may explain our null results. Gan et al. analyzed BOLD signals when participants learned they would play the high or low provocation opponent. We examined BOLD responses during decision-making and aggressive behavior. Similarly, we did not find evidence that high versus low provocation modulated BOLD responses. Because we analyzed the decision phase, participants may have already decided what level to select during the anticipation phase.
In addition to decreased activity in the caudate and ventral striatum among intoxicated participants, we also found greater hippocampal activity in intoxicated participants relative to sober participants. Regardless, our results are largely consistent with a growing body of research on the neural correlates of aggression that implicate altered functioning in the PFC, the limbic system, and reward-related regions (Achterberg et al., 2016; Beyer et al., 2014; Chester & DeWall, 2016; Emmerling et al., 2016; Krämer et al., 2007; Lotze et al., 2007), and more recently, alcohol-related aggression (Gan et al., 2015; Kose et al., 2015).
The present results are consistent with several psychological theories of alcohol-related aggression. The attention-allocation model suggests that alcohol limits the amount of information that can be processed in working memory (Giancola, Josephs, Parrott, & Duke, 2010). Aggression is thought to occur because alcohol focuses attention on instigatory cues (such as the noise blasts) and away from inhibitory cues (norms proscribing aggression). When sober, people are able to process both types of cues (e.g., Denson et al., 2008). A meta-analysis reported that the dorsolateral PFC is activated when managing content in working memory (Owen, McMillan, Laird, & Bullmore, 2005). Thus, the alcohol-induced reduction in dorsolateral PFC activity may have made it more difficult for intoxicated than for sober participants to manage both instigatory and inhibitory cues in working memory. It is also possible that the dorsolateral PFC in intoxicated participants may only process instigatory cues. Similarly, although the hippocampus is generally not associated with working memory (Owen et al., 2005), perhaps greater difficulty incorporating cues into episodic memory was the cause of heightened hippocampal activation that we observed among intoxicated participants (Henke, Buck, Weber, & Wieser, 1997).
Complementing the attention-allocation model, hostile attribution theory suggests that alcohol-related aggression occurs because alcohol makes relatively innocuous and ambiguous cues more likely to be interpreted as provocative (Bartholow & Heinz, 2006; Subra, Muller, Bègue, Bushman, & Delmas, 2010). Thus, the positive relationships between the dorsomedial and dorsolateral PFC activity and aggressive behavior in intoxicated participants may be due to biased processing toward hostile cues. Indeed, the dorsomedial PFC is associated with angry rumination and mentalizing about provocations (Denson, Pedersen, Ronquillo, & Nandy, 2009; Lotze et al., 2007). We found substantial overlap in the medial PFC between this study and our previous study of angry rumination (Denson et al., 2009).
Because the dorsomedial PFC is broadly associated with introspective processes in sober participants, the reduction in dorsomedial PFC activity in intoxicated participants may reflect reduced self-awareness. According to one major theory of alcohol-related aggression, self-awareness is thought to remain intact and inhibit aggression when sober but become impaired in intoxicated people (Ito et al., 1996). This loss of self-awareness when intoxicated is thought to facilitate aggression because people lose track of their personal and normative standards. Indeed, meta-analytic work shows that increasing self-awareness can reduce aggression in participants who consumed low doses of alcohol (Ito et al., 1996). Future research could test the hypothesis that dorsomedial PFC activation in intoxicated participants reflects vengeful rumination and lower self-awareness, whereas dorsomedial PFC activation is sober participants may reflect intact self-awareness. Thus, our findings are consistent with four major theories of alcohol-related aggression: the PFC dysfunction model, the attention-allocation model, hostile attribution bias theory, and self-awareness theory. However, one aspect of our data that these theories do not explain is why, despite a mean-level alcohol-induced reduction in PFC activity, we still observed a positive correlation between PFC activity and aggressive behavior in the alcohol group. Provided future research replicates these findings, researchers may wish to incorporate these and other neuroimaging findings into theories of alcohol-related violence.
Another perspective is that our results are consistent with a growing body of evidence showing that the PFC not only “puts the brakes” on aggressive behavior (Raine, 2008) but also that the PFC can facilitate aggression (as it did for intoxicated participants). Like other forms of goal-directed behavior, the PFC is responsible for integrating and enacting this behavior. For instance, a recent study found that disruption to the dorsolateral PFC with repetitive transcranial magnetic stimulation reduced punishment for criminal acts (Buckholtz et al., 2015). Moreover, this reduced punishment was because disruption to the dorsolateral PFC kept participants from integrating culpability and harm judgments. Thus, the dorsolateral PFC is necessary for enacting some forms of aggression but also for inhibiting aggression. Our findings suggest that there is probably no simple mediating effect of reduced PFC activity on the relationship between alcohol and aggression. Rather, PFC activation in intoxicated and sober participants likely facilitates aggression via discriminant psychological processes.
Limitations
The present research was limited in some aspects. Our sample is probably qualitatively different from many perpetrators of severe alcohol-related violence. The sample consisted of young male social drinkers without significant histories of violence. Much alcohol-related violence is disproportionately perpetrated by men with alcohol-use disorders and men with violent traits such as antisocial personality disorder. Thus, our sample is unlikely to be at high risk of alcohol-related violence. Nonetheless, future neuroimaging research could examine the neural correlates of alcohol-related aggression in women and men predisposed toward alcohol-related violence. Indeed, in prior studies using the TAP, men and women both demonstrated intoxicated aggression (Duke et al., 2011; Gan et al., 2015). Until further research is conducted, our results may not generalize to heavy drinkers, women, and those with strong impelling factors, such as antisocial personality disorder. However, because neuroimaging research on alcohol-related violence is in its infancy, we chose to begin with a sample of healthy men to control extraneous variance and help form the basis for future work with clinical samples.
We used a mixed design, with condition as a between-subjects factor rather than a completely within-subjects design. Both designs are informative yet limited in some respects. For a minority of people, believing that they have consumed alcohol increases aggression. This concern is more likely to be eliminated in within-subjects designs. In the present research, participants in the placebo condition thought they consumed 2.86 standard drinks on average compared to 3.65 standard drinks for participants in the alcohol group. Thus, aggressive expectancies around perceived alcohol consumption combined with a relatively low dose of alcohol may explain why there were no significant differences in aggression between groups. Nonetheless, the effect of alcohol on aggression was in the expected direction and comparable in size (d = 0.20) to placebo-controlled alcohol-aggression experiments that utilized relatively low doses of alcohol such as ours (Malcohol = 2.61, SD = 0.66; Mplacebo = 2.44, SD = 0.95; see the meta-analysis by Ito et al., 1996). Moreover, the sample size was modest. Extension to larger samples and violent offenders using multiple doses of alcohol will facilitate understanding of the devastating and costly social phenomenon of alcohol-related violence. Future work could examine additional design features, such as no-drink or mixed energy drink conditions. We also did not use arterial spin labeling MRI to assess how alcohol might have influenced cerebral perfusion and BOLD responses during the TAP (e.g., Gan et al., 2015; Marxen et al., 2014). Finally, some recent research has extended the TAP response options from 4 to 8, which may provide more sensitivity (Beyer et al., 2014; Gan et al., 2015).
Conclusion
Even at a low dose of alcohol, we observed a significant relationship between dorsomedial and dorsolateral PFC activity and alcohol-related aggression. These findings corroborate the predictions of many of the major theories of intoxicated aggression. We encourage future, larger-scale investigations into the neural underpinnings of alcohol-related aggression with stronger doses and clinical samples. Doing so could eventually substantially reduce alcohol-related harm.
Notes
One participant with an AUDIT score of 18 was erroneously admitted to the study.
Because other fMRI studies have found correlations between striatum activation and aggressive behavior, we also conducted a post hoc correlation analysis between the ventral striatum cluster that was activated by the alcohol manipulation and aggressive behavior on the TAP. In the whole sample, this analysis showed a significant positive correlation between a small cluster in the left striatum and aggressive behavior, r(48) = .31, p = .030, q(FDR) < .05, peak activation x = −19, y = 2, z = −2, five contiguous voxels. The correlations were in the same positive direction but not individually significant in the placebo condition, r(25) = .34, p = .08, or alcohol condition, r(21) = .34, p = .11.
References
Achterberg, M., van Duijvenvoorde, A. C., Bakermans-Kranenburg, M. J., & Crone, E. A. (2016). Control your anger! The neural basis of aggression regulation in response to negative social feedback. Social Cognitive and Affective Neuroscience, 11, 712–720.
Aiken, L. S., West, S. G., & Reno, R. R. (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage.
Anderson, B. M., Stevens, M. C., Meda, S. A., Jordan, K., Calhoun, V. D., & Pearlson, G. D. (2011). Functional imaging of cognitive control during acute alcohol intoxication. Alcoholism: Clinical and Experimental Research, 35, 156–165.
Bartholow, B. D., & Heinz, A. (2006). Alcohol and aggression without consumption alcohol cues, aggressive thoughts, and hostile perception bias. Psychological Science, 17(1), 30–37.
Beyer, F., Münte, T. F., Erdmann, C., & Krämer, U. M. (2014). Emotional reactivity to threat modulates activity in mentalizing network during aggression. Social Cognitive and Affective Neuroscience, 9(10), 1552–1560.
Buckholtz, J. W., Martin, J. W., Treadway, M. T., Jan, K., Zald, D. H., Jones, O., & Marois, R. (2015). From blame to punishment: Disrupting prefrontal cortex activity reveals norm enforcement mechanisms. Neuron, 87(6), 1369–1380.
Bushman, B. J. (1993). Human aggression while under the influence of alcohol and other drugs: An integrative research review. Current Directions in Psychological Science, 2, 148–151.
Bushman B. J., & Cooper H. (1990). Effects of alcohol on human aggression: An integrative research review. Psychological Bulletin, 107, 341–354.
Buss, A. H., & Perry, M. P. (1992). The Aggression Questionnaire. Journal of Personality and Social Psychology, 63, 452–459.
Cherek, D. R. (1981). Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology, 75, 339–345.
Chester, D. S., & DeWall, C. N. (2016). The pleasure of revenge: Retaliatory aggression arises from a neural imbalance toward reward. Social Cognitive and Affective Neuroscience, 11, 1173–1182.
Chester, D. S., Eisenberger, N. I., Pond, R. S., Richman, S. B., Bushman, B. J., & DeWall, C. N. (2013). The interactive effect of social pain and executive functioning on aggression: An fMRI experiment. Social Cognitive and Affective Neuroscience, 9, 699–704.
Dambacher, F., Sack, A. T., Lobbestael, J., Arntz, A., Brugman, S., & Schuhmann, T. (2015a). Out of control: Evidence for anterior insula involvement in motor impulsivity and reactive aggression. Social Cognitive and Affective Neuroscience, 10(4), 508–516.
Dambacher, F., Schuhmann, T., Lobbestael, J., Arntz, A., Brugman, S., & Sack, A. T. (2015b). Reducing proactive aggression through non-invasive brain stimulation. Social Cognitive and Affective Neuroscience, 10, 1303–1309.
Denson, T. F., Aviles, F. E., Pollock, V. E., Earleywine, M., Vasquez, E. A., & Miller, N. (2008). The effects of alcohol and the salience of aggressive cues on triggered displaced aggression. Aggressive Behavior, 34(1), 25–33.
Denson, T. F., DeWall, C. N., & Finkel, E. J. (2012). Self-control and aggression. Current Directions in Psychological Science, 21, 20–25.
Denson, T. F., Pedersen, W. C., Ronquillo, J., & Nandy, A. S. (2009). The angry brain: Neural correlates of anger, angry rumination, and aggressive personality. Journal of Cognitive Neuroscience, 21, 734–744.
Duke, A. A., Giancola, P. R., Morris, D. H., Holt, J. C., & Gunn, R. L. (2011). Alcohol dose and aggression: Another reason why drinking more is a bad idea. Journal of Studies on Alcohol and Drugs, 72, 34–43.
Easdon, C. M., & Vogel-Sprott, M. (2000). Alcohol and behavioral control: Impaired response inhibition and flexibility in social drinkers. Experimental Clinical Psychopharmacology, 8, 387–394.
Emmerling, F., Schuhmann, T., Lobbestael, J., Arntz, A., Brugman, S., & Sack, A. T. (2016). The role of the insular cortex in retaliation. PLOS ONE, 11(4), e0152000.
Fals-Stewart, W., Leonard, K. E., & Birchler, G. R. (2005). The occurrence of male-to-female intimate partner violence on days of men’s drinking: The moderating effects of antisocial personality disorder. Journal of Consulting and Clinical Psychology, 73, 239–248.
Finkel, E. J., & Hall, A. N. (2017). The I3 model: A metatheoretical framework for understanding aggression. Current Opinion in Psychology, 19, 125–130. https://doi.org/10.1016/j.copsyc.2017.03.013
Finn, P. R., Justus, A., Mazas, C. & Steinmetz, J. E. (1999). Working memory, executive processes and the effects of alcohol on go/no-go learning: Testing a model of behavioral regulation and impulsivity. Psychopharmacology, 146, 465–472.
Foran, H. M., & O’Leary, K. D. (2008). Alcohol and intimate partner violence: A meta-analytic review. Clinical Psychology Review, 28(7), 1222–1234.
Gan, G., Guevara, A., Marxen, M., Neumann, M., Jünger, E., Kobiella, A., ... & Smolka, M. N. (2014). Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biological Psychiatry, 76(9), 698–707.
Gan, G., Sterzer, P., Marxen, M., Zimmermann, U. S., & Smolka, M. N. (2015). Neural and behavioral correlates of alcohol-induced aggression under provocation. Neuropsychopharmacology, 40, 2886–2896.
Giancola, P. R. (2000). Executive functioning: A conceptual framework for alcohol-related aggression. Experimental and Clinical Psychopharmacology, 8, 576.
Giancola, P. R., Josephs, R. A., Parrott, D. J., & Duke, A. A. (2010). Alcohol myopia revisited clarifying aggression and other acts of disinhibition through a distorted lens. Perspectives on Psychological Science, 5, 265–278.
Gorka, S. M., Fitzgerald, D. A., King, A. C., & Phan, K. L. (2013). Alcohol attenuates amygdala–frontal connectivity during processing social signals in heavy social drinkers. Psychopharmacology, 229, 141–154.
Graham, K. (1980). Theories of intoxicated aggression. Canadian Journal of Behavioural Science/Revue canadienne des sciences du comportement, 12, 141–158.
Guillot, C. R., Fanning, J. R., Bullock, J. S., McCloskey, M. S., & Berman, M. E. (2010). Effects of alcohol on tests of executive functioning in men and women: A dose response examination. Experimental Clinical Psychopharmacology, 18, 409–417.
Heinz, A. J., Beck, A., Meyer-Lindenberg, A., Sterzer, P., & Heinz, A. (2011). Cognitive and neurobiological mechanisms of alcohol-related aggression. Nature Reviews Neuroscience, 12, 400–413.
Henke, K., Buck, A., Weber, B., & Wieser, H. G. (1997). Human hippocampus establishes associations in memory. Hippocampus, 7, 249–256.
Hoaken, P. N., & Stewart, S. H. (2003). Drugs of abuse and the elicitation of human aggressive behavior. Addictive Behaviors, 28(9), 1533–1554.
Hull, J., & Bond, C. (1986). Social and behavioral consequences of alcohol consumption and expectancy: A meta-analysis. Psychological Bulletin, 99, 347–360.
Ito, T., Miller, N., & Pollock, V. (1996). Alcohol and aggression: A meta-analysis on the moderating effects of inhibitory cues, triggering events, and self-focused attention. Psychological Bulletin, 120, 60–82.
Kareken, D. A., Liang, T., Wetherill, L., Dzemidzic, M., Bragulat, V., Cox, C., ... & Foroud, T. (2010). A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcoholism: Clinical and Experimental Research, 34(12), 2169–2178.
Kose, S., Steinberg, J. L., Moeller, F. G., Gowin, J. L., Zuniga, E., Kamdar, Z. N., ... & Lane, S. D. (2015). Neural correlates of impulsive aggressive behavior in subjects with a history of alcohol dependence. Behavioral neuroscience, 129(2), 183–196.
Krämer, U. M., Jansma, H., Tempelmann, C., & Münte, T. F. (2007). Tit-for-tat: The neural basis of reactive aggression. NeuroImage, 38, 203–211.
Krämer, U. M., Riba, J., Richter, S., & Münte, T. F. (2011). An fMRI study on the role of serotonin in reactive aggression. PLOS ONE, 6(11), e27668.
Lotze, M., Veit, R., Anders, S., & Birbaumer, N. (2007). Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. NeuroImage, 34, 470–478.
Lyvers, M. F., & Maltzman, I. (1991). Selective effects of alcohol on Wisconsin card sorting test-performance. British Journal of Addiction, 86, 399–407.
Marxen, M., Gan, G., Schwarz, D., Mennigen, E., Pilhatsch, M., Zimmermann, U. S., ... & Smolka, M. N. (2014). Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. Journal of Cerebral Blood Flow & Metabolism, 34, 472–479.
Miczek, K. A., Barros, H. M., Sakoda, L., & Weerts, E. M. (1998). Alcohol and heightened aggression in individual mice. Alcoholism: Clinical and Experimental Research, 22, 1698–1705.
Murdoch, D., & Ross, D. (1990). Alcohol and crimes of violence: Present issues. International Journal of the Addictions, 25(9), 1065–1081.
Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25, 46–59.
Parrott, D. J., & Zeichner, A. (2002). Effects of alcohol and trait anger on physical aggression in men. Journal of Studies on Alcohol, 63, 196–204.
Peterson, J. B., Rothfleisch, J., Zelazo, P. D., & Pihl, R. O. (1990). Acute alcohol-intoxication and cognitive functioning. Journal of Studies on Alcohol, 51, 114–122.
Quigley, B. M., & Leonard, K. E. (2000). Alcohol, drugs, and violence. In V. B. Van Hasselt & M. Hersen (Eds.), Aggression and violence: An introductory text (pp. 259–283). Needham Heights, MA: Allyn & Bacon.
R Core Team. (2016). R: A language and environment for statistical computing [Computer software]. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/
Raine, A. (2008). From genes to brain to antisocial behavior. Current Directions in Psychological Science, 17, 323–328.
Riva, P., Romero Lauro, L. J., DeWall, C. N., Chester, D. S., & Bushman, B. J. (2015). Reducing aggressive responses to social exclusion using transcranial direct current stimulation. Social cognitive and affective neuroscience, 10, 352–356.
Room, R., & Rossow, I. (2001). The share of violence attributable to drinking. Journal of Substance Use, 6, 218–228.
Schofield, T. P., Unkelbach, C., & Denson, T. F. (2017). Alcohol consumption increases the bias to shoot at Middle Eastern but not White targets. Group Processes & Intergroup Relations, 20, 202–215.
Schreckenberger, M., Amberg, R., Scheurich, A., Lochmann, M., Tichy, W., Kiega, A., ... Urban, R. (2004). Acute alcohol effects on neuronal and attentional processing: Striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology 29, 1527–1537.
Smith, S., Jenkinson, M., Woolrich, M., Beckmann, C., Behrens, T., Johansen-Berg, H., ... Matthews, P. M. (2004). Advances in structural and functional MR image analysis and implementation in FSL. NeuroImage, 23, 208–209.
Steele, C. M., & Southwick, L. (1985). Alcohol and social behavior: I. The psychology of drunken excess. Journal of Personality and Social Psychology, 48, 18–34.
Subra, B., Muller, D., Bègue, L., Bushman, B. J., & Delmas, F. (2010). Automatic effects of alcohol and aggressive cues on aggressive thoughts and behaviors. Personality and Social Psychology Bulletin, 36, 1052–1057.
Swanson, J. W., Holzer, C. E., III, Ganju, V. K., & Jono, R. T. (1990). Violence and psychiatric disorder in the community: Evidence from the Epidemiologic Catchment Area surveys. Psychiatric Services, 41, 761–770.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme.
Taylor, S. P. (1967). Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. Journal of Personality, 35, 297–310.
U.S. Department of Justice. (1998). Alcohol and crime: An analysis of national data on the prevalence of alcohol involvement in crime. Retrieved from http://www.bjs.gov/content/pub/pdf/ac.pdf
WHO. (2006). Interpersonal violence and alcohol. Retrieved from http://www.who.int/violence_injury_prevention/violence/world_report/factsheets/pb_violencealcohol.pdf
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Denson, T.F., Blundell, K.A., Schofield, T.P. et al. The neural correlates of alcohol-related aggression. Cogn Affect Behav Neurosci 18, 203–215 (2018). https://doi.org/10.3758/s13415-017-0558-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-017-0558-0