Abstract
Processing within the dorsal visual stream subserves object-directed action, whereas visual object recognition is mediated by the ventral visual stream. Recent findings suggest that the computations performed by the dorsal stream can nevertheless influence object recognition. Little is known, however, about the type of dorsal stream information that is available to assist in object recognition. Here, we present a series of experiments that explored different psychophysical manipulations known to bias the processing of a stimulus toward the dorsal visual stream in order to isolate its contribution to object recognition. We show that elongated-shaped stimuli, regardless of their semantic category and familiarity, when processed by the dorsal stream, elicit visuomotor grasp-related information that affects how we categorize manipulable objects. Elongated stimuli may reduce ambiguity during grasp preparation by providing a coarse cue to hand shaping and orientation that is sufficient to support action planning. We propose that this dorsal-stream-based analysis of elongation along a principal axis is the basis for how the dorsal visual object processing stream can affect categorization of manipulable objects.
Similar content being viewed by others
Introduction
A long tradition of research in cognitive science has dissected out the components of object processing within the ventral and dorsal visual pathways. Ventral visual regions, including ventral and lateral occipito-temporal cortices, mediate processing of shape, color, and texture information in support of visual object recognition (e.g., Cant & Goodale, 2007; Goodale & Milner, 1992; Grill-Spector, Kourtzi, & Kanwisher, 2001; Miceli, Fouch, Capasso, Shelton, Tamaiuolo, & Caramazza, 2001). The dorsal visual stream, which projects from V1 through dorsal occipital to posterior parietal structures, is responsible for spatial and visuomotor analyses necessary for grasping and manipulating objects (e.g., Culham, Danckert, Souza, Gati, Menon, & Goodale, 2003; Goodale & Milner, 1992; Johnson-Frey, 2004).
Insofar as visual object recognition is concerned, processes mediated by the dorsal visual pathway typically have not been regarded as an important, or even a potential, source of information (e.g., Goodale & Milner, 1992; Miller, Nieder, Freedman, & Wallis, 2003; for a review, see Mahon & Caramazza, 2005). Recently, however, it has become clear that dorsal stream visuomotor information can interact with conceptual decisions about objects (e.g., Almeida, Mahon, & Caramazza, 2010; Almeida, Mahon, Nakayama, & Caramazza, 2008; Helbig, Graf, & Kiefer, 2006). Here, we will address the nature of the dorsal stream information that can affect the conceptual analysis of manipulable objects.
Most discussions of the dorsal visual pathway emphasize its role in visually guided action; neurophysiological and neuroimaging studies have shown that the dorsal stream is highly tuned to grasp and reach preparation (e.g., Culham et al., 2003; Murata, Gallese, Luppino, Kaseda, & Sakata, 2000) and online control of movements (e.g., Desmurget et al., 1999). Hence, the kind of object-related information that the dorsal stream may contribute to conceptual decision making should be related to visuomotor processing about volumetrically optimal grasps and, in general, to the grasping status of an object.
A structural—and typically, visual—dimension that may facilitate the visuomotor description of an object and, as such, be useful in selecting a particular grasp is object elongation. Elongation is a coarse cue for object graspability. The majority of the handheld tools that we use in our daily life have an elongated shape with one major and unambiguous longitudinal axis. Moreover, elongated shapes have spatial characteristics that facilitate the preparation of a particular grasp—for example, the presence of a handle. As such, elongation is a visual dimension that could have a privileged relationship to processing within the dorsal visual pathway. The experiments reported below test whether elongation, as a visual dimension, can affect conceptual decisions about manipulable objects.
Experiments
If object elongation is a stimulus dimension that “triggers” processing by the dorsal stream, which can then affect conceptual decisions about manipulable objects, elongated items, regardless of their category and when processed differentially by the dorsal stream, should influence the categorization of manipulable objects. To address this issue, we manipulated whether primes were elongated, independently of their semantic category membership. For instance, we presented an image of a fish (i.e., an elongated object that is an animal) and of a knife (i.e., an elongated object that is a tool). The processing of the image of a fish may elicit some ambiguity in the visual system: The ventral stream may perceive it as an animal and, hence, quite different from a knife, whereas processing within the dorsal stream may be triggered by the fact that it is an elongated object and, as such, “invites” a particular grasp—in part, similar to the dorsal stream’s “understanding’ of a knife. Thus, the issue is whether, when used as a prime and in situations where processing is biased toward dorsal stream structures, fish behaves like knife in priming the categorization of tool targets, as compared with a prime image of an elephant (i.e., a nonelongated animal). In order to explore this question, we used a series of psychophysical manipulations to bias the processing of unconsciously presented prime pictures toward the dorsal visual stream.
In Experiments 1 and 2, we exploited the processing characteristics of two different psychophysical procedures: continuous flash suppression (CFS; e.g., Tsuchiya & Koch, 2005), and backward masking (BM; e.g., Breitmeyer & Ogmen, 2000). Pictures rendered invisible by these procedures seem to activate different parts of the visually responsive cortex. On the one hand, pictures suppressed under CFS (and, in particular, pictures of tools) elicit activity within parietal and occipito-parietal regions of the dorsal stream that is comparable to that obtained for pictures presented visibly, whereas activity within the ventral visual stream is dramatically reduced under CFS, as compared with visible conditions (e.g., Fang & He, 2005; Logothetis & Schall, 1989; see also Tong, Nakayama, Vaughan, & Kanwisher, 1998). Note, however, that these data have recently been challenged: Some authors have failed to report any neural activity (irrespective of the visual stream) for CFS-suppressed images (e.g., Hesselmann & Malach, 2011), whereas others have shown that information can be decoded about CFS-suppressed images from ventral stream structures with the use of more refined analysis (i.e., multi-voxel pattern analysis; Sterzer, Haynes, & Rees, 2008). Overall, though, the data on the neural fate of CFS-suppressed information suggest that while claims about strong dissociations between dorsal and ventral visual streams under CFS stimulation are not sustainable, there are grounds for assuming that CFS leads to a relative bias in processing for the dorsal, as compared with the ventral, stream.
On the other hand, pictures presented under BM elicit appreciable neural responses in a much wider set of brain regions, including regions within both ventral and dorsal visual streams (Dehaene et al., 2001; Rolls & Tovee, 1994). Thus, the information computed from CFS-suppressed stimuli and stimuli rendered invisible with BM will differ, principally, in the extent to which the processing within the dorsal stream is relatively isolated or is accompanied by processing within the ventral stream, respectively (for a similar approach, see Almeida et al., 2010; Almeida et al., 2008). We can therefore predict that elongated primes (e.g., fish, knife) rendered invisible with CFS will facilitate processing of a tool target. However, when BM is used to render primes invisible, we predict that it is the category membership of the prime that will govern priming effects.
In Experiment 3, we used BM and explored hemispheric asymmetries in the processing of manipulable objects (for a similar approach, see Garcea, Almeida, & Mahon, 2012). The neural processing of manipulable objects is strongly left-lateralized within dorsal stream structures. When pictures of tools, as compared with pictures of animals, are viewed, a left-lateralized network of regions including inferior and superior parietal regions and the ventral premotor cortex is activated (e.g., Chao & Martin, 2000; Johnson-Frey, Newman-Norland, & Grafton, 2005; Mahon et al., 2007; Noppeney, Price, Penny, & Friston, 2006; for reviews, see Lewis, 2006; Martin, 2007). Moreover, brain damage affecting left-hemisphere parietal structures or the left lateral temporal cortex can lead to impairments in the knowledge of how to manipulate tools and/or to conceptual impairments for tools (Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004; Johnson-Frey, 2004; Mahon et al., 2007; Tranel, Damasio, & Damasio, 1997). Interestingly, that asymmetry is not as apparent within ventral stream structures; viewing tools, in comparison with other categories, typically leads to bilateral activations of the medial fusiform gyrus (e.g., Chao & Martin, 2000; Mahon et al., 2007), although the effect is often stronger in the left than in the right. Handy, Grafton, Shroff, Ketay, and Gazzaniga (2003) showed that tool-related visuomotor information (e.g., affordances) influenced participants’ performance when presented in the right and lower visual fields—suggesting a left-hemisphere dorsal stream locus. This result was further backed up by their fMRI results, showing strong left-lateralization within parietal and premotor regions for the processing of the affordances provided by tool stimuli.
There is a strong tradition of using procedures that present stimuli to the left visual field (LVF) and/or the right visual field (RVF) (and crucially, away from the fovea) to explore hemispheric asymmetries in other domains (e.g., Bub & Lewine, 1988; Chiarello, Nuding, & Pollock, 1988; Finkbeiner, Almeida, & Caramazza, 2006; Garcea et al., 2012; Hunter & Brysbaert, 2008). The feasibility of these procedures rests on the fact that the LVF projects to the right hemisphere, whereas the RVF projects to the left hemisphere, and on the assumption that projecting directly to a functionally specialized network will lead to more efficient processing of information. Given that, for tools, there is strong left-lateralization in the dorsal, but not ventral, visual pathways, Experiment 3 used RVF presentations to present stimuli to both the dorsal and ventral visual pathways and LVF presentations to bias processing toward ventral stream structures. As such, we anticipated that the ambiguity in the processing of a picture of an elongated fish would lead to faster responses for tool targets when this prime picture was presented in the RVF, but not when it was presented in the LVF. This is because RVF presentations would favor an interpretation of a fish as an elongated object over the interpretation as an animal (or at the very least, would offer such an interpretation along with the category membership), whereas LVF presentation would not lead to this bias in interpretation (and if anything, would lead to the opposite). That is, responses for tool targets should be affected by an elongated animal prime picture when presented in the RVF, but not when presented in the LVF, whereas tool primes should facilitate tool target categorization irrespective of the visual field in which they are presented.
Finally, in Experiment 4, we employed BM to mask our prime pictures and measured reaching movements (e.g., Finkbeiner & Friedman, 2011; Song & Nakayama, 2008), since this dependent measure relies on processing that takes place within dorsal stream structures. The involvement of dorsal stream structures in planning and executing reaching movements has been widely demonstrated. For instance, online visuomotor corrections that are crucial for the execution of reaching movements are performed by the dorsal visual stream (e.g., Desmurget et al., 1999; Goodale, Pelisson, & Prablanc, 1986). Moreover, single-cell recording studies in nonhuman primates have strongly associated the planning of reaching movements with a particular group of regions within the posterior parietal cortex (i.e., the parietal reach area; e.g., Batista, Buneo, Snyder, & Andersen, 1999). Neuroimaging and neuropsychological human data corroborate these findings. Patients suffering from optic ataxia following lesions to the posterior-superior parietal cortex have difficulty reaching toward targets (e.g., Perenin & Vighetto, 1988), particularly when these are presented in the periphery. Human fMRI data from healthy participants have shown that regions in the vicinity of the intraparietal sulcus are involved in reaching tasks (e.g., Connolly, Andersen, & Goodale, 2003; Prado et al., 2005; for a review, see Culham, Cavina-Pratesi & Singhal 2006). In Experiment 4, we asked participants to reach and point toward an area of the screen, the location of which was dependent on the category of the target. Because the analyses of reaching trajectories may provide a more direct view of dorsal-stream-based effects than do traditional buttonpress measures, we predicted that in this experiment, elongated animal primes would behave more like elongated tool primes in modulating categorization of tool targets than would nonelongated animal primes. That is, in this experiment, we changed the type of response requested (from the typical buttonpress to reaching trajectories), rather than the presentation technique or the position of the stimulus (as in the previous experiments), and expected that this response type, because of its reliance on dorsal stream processing, would reveal the effects of a bias for elongated stimuli to be processed by dorsal stream structures.
To anticipate our results, elongated primes, when presented under conditions that bias processing toward the dorsal stream, lead to priming effects for tool targets, irrespective of the semantic category of the prime. This pattern of results suggests that dorsal stream information pertaining to a visuomotor dimension—object elongation—restricts the pool of alternative object-related visuomotor descriptions and can affect conceptual decisions.
Experiment 1
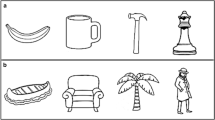
We asked participants to perform a simple categorization task on target pictures that belonged to the category of either tools or animals. Participants were asked to press a response key with one index finger if the target was an animal and to press another key with the other index finger if the target was a tool. Unbeknownst to participants, each target picture was preceded by a prime picture that could also be an animal or a tool. Within each prime category, we chose items that had either an elongated shape (e.g., hammer, fish), or a “blob-like” shape (e.g., doorknob, horse; see Fig. 1a). We did not manipulate the elongation profile of the target pictures. Rather, we used typical animals, which tend to be blob-like, and typical tools, which tend to be elongated (see below for further discussion).
Stimuli and experiment design. a Examples of the prime stimuli used in Experiments 1–4. b For Experiments 1a and 2, we used continuous flash suppression to render the prime stimuli invisible. c For Experiments 1b and 4, we used backward masking (BM) to render the prime stimuli invisible. Note that for Experiment 3, we used BM but prime presentation was lateralized
Two different procedures were used to present the prime pictures and render them invisible: CFS (Experiment 1a; see Fig. 1b) and BM (Experiment 1b; see Fig. 1c). In CFS, a static image competes (interocularly) with a dynamic pattern, with the latter reliably suppressing the former for a prolonged period of time, whereas in BM, an image is presented for a brief amount of time (e.g., 20 ms), followed immediately by a high-contrast random-noise mask that renders the briefly presented image invisible.
Method
Participants
One hundred four undergraduate students participated in the study in exchange for course credit or payment (54 in Experiment 1a and 50 in Experiment 1b). All participants had normal or corrected-to-normal vision, were right-handed, and gave written informed consent. Participants were naïve as to the experimental hypotheses. The project was approved by the appropriate institutional review board.
Materials and procedure
We used black-and-white pictures of animals and tools. For each semantic category, we selected 4 pictures as targets and 8 different pictures as primes (see supplementary Fig. 1 for the full set of pictures; for examples, see the first and second columns of Fig. 1a). Half of the prime pictures within each semantic category had an elongated shape, whereas the other half had a blob-like shape. In total, 16 prime pictures were selected: four blob-like animals (elephant, turtle, cow, and spider), four elongated animals (eel, dragonfly, caterpillar, and fish), four blob-like “tools” (faucet knob, doorknob, tape measure, and shower sponge), and four elongated tools (hammer, saw, hairbrush, and wrench). This way, the primes were categorically congruent with one group of targets and categorically incongruent with the other (e.g., animal primes were categorically congruent with animal target pictures and categorically incongruent with tool target pictures) and could orthogonally belong to one of the two different shape categories (i.e., elongated and blob-like). Note that the target pictures of tools that we used were all elongated in shape, whereas the target pictures of animals were all blob-like in shape. The same pictures were used in Experiments 1a and 1b. Each participant completed 384 trials, with each of the eight targets being presented 48 times and each of the 16 primes being presented 24 times.
In Experiment 1a, the primes were rendered invisible using CFS (see Fig. 1b). To induce CFS, red/green anaglyph glasses were worn by the participants to allow for dichoptic presentation of the images. On each trial, we presented a low-luminance, low-contrast version of the prime stimulus, restricted to the green RGB channel, to the participant’s nondominant eye and a dynamic high-contrast random-noise pattern, restricted to the red RGB channel, that changed every 100 ms to the dominant eye (for further discussion, see Almeida et al., 2010; Almeida et al., 2008). The stimuli were presented centrally and subtended 7° of visual angle. In this experiment, participants saw a fixation cross (for 500 ms), the prime and the first random-noise pattern (for 100 ms), the prime and a second random-noise pattern (100 ms), and finally the target picture, for 3 s or until the participant responded (whichever came first; see Fig. 1b). Participants categorized the target stimuli as an animal or a tool by means of a buttonpress response as quickly and accurately as possible.
In Experiment 1b, prime pictures were rendered invisible via BM (see Fig. 1c), in which a briefly presented prime picture is followed by a high-contrast backward mask. Participants saw a fixation cross (for 500 ms), then the prime picture (for 20 ms), the backward mask (100 ms), and finally the target picture, for 3 s or until the participant responded (whichever came first; see Fig. 1c). As in Experiment 1a, participants were asked to categorize the target stimuli as quickly and accurately as possible.
In both Experiments 1a and 1b, after the experiment proper, participants performed a prime discrimination task. The prime discrimination task provided independent data to ensure lack of awareness of the prime. In this task, participants were informed of the presence of a prime and were instructed to categorize the primes as either animals or tools. The trial sequence remained the same as in the previous tasks, except that the target was not presented. In Experiment 1a, participants performed the prime discrimination task for primes rendered invisible with CFS, whereas in Experiment 1b, prime discrimination was carried out for primes rendered invisible using BM conditions. Only participants who performed at chance on the prime discrimination task were included in the main analyses reported for both experiments (see Fig. S2a, b for more detailed analyses).
All experiments were run on a Dell PC, with a ViewSonic ultrabrite A90_f monitor. The monitor refresh rate was 100 Hz. Stimuli were presented using DisplayMaster DirectX (DMDX) software (Forster & Forster, 2003). Experiments 1a and 1b each lasted approximately 40 min.
Analysis
Planned contrasts were used to analyze response latencies (Rosenthal, Rosnow, & Rubin, 2000). For each category in each experiment, three pairwise contrasts were employed to test for a priming effect in categorization responses times. The priming effect was calculated as the difference in response time between incongruent trials and the other conditions. For animal targets, elongated tools were considered as the canonical incongruent condition and were contrasted against the other prime conditions (i.e., blob-like animals, elongated animals, and blob-like “tools”). For tool targets, blob-like animals were considered as the canonical incongruent prime condition and were compared with all the other prime conditions (i.e., elongated tools, blob-like “tools,” and elongated animals).
For the prime awareness task, participants who reported seeing any prime during the experiment proper or prime awareness task were immediately discarded without further analysis. The remaining data were entered into a z-test for one proportion. Participants whose global data were significantly different from chance (at p < .05) were discarded. We also tested whether there were differences in prime awareness between the prime conditions using a z-test for two proportions; participants showing significant differences were also not included in the main analysis.
Results
For both BM and CFS, an independent prime awareness measurement task was used to select participants who could not reliably report the category of the prime (i.e., who were objectively and subjectively unaware of the prime pictures; see Table 1 and Fig. S1). In Experiment 1a, 2 participants were discarded because their error rate was above 2 standard deviations from the mean error rate of the participants. Of the remaining 52 participants, 27 participants were included in the analysis because they were not objectively and/or subjectively aware of the primes in the prime awareness task. In Experiment 1b, 29 out of the 50 participants were included in the main analysis, since they were not objectively and/or subjectively aware of the primes.
For both procedures, we obtained priming results that demonstrated that the primes were processed and were used during target categorization (for mean response times, see Table 2). The results were, however, dramatically different for the two procedures. For the CFS experiment (Experiment 1a; N = 27), elongated tool (e.g., hammer) and elongated animal (e.g., fish) primes elicited faster categorization responses for tool targets, when compared with blob-like animal primes [e.g., cow; average priming for elongated tool = 12 ms, SEM = 4 ms, t(26) = 3.08, p = .005; average priming for elongated animals = 8 ms, SEM = 3 ms, t(26) = 2.43, p = .022; for blob-like “tools,” t < 1; Fig. 2a]. In contrast, no effects of the prime pictures were obtained for the categorization of animal targets [ts < 1; for blob-like “tool” prime stimuli, t(26) = 1.53, p = .139; Fig 2a].
Behavioral priming effects for Experiment 1. Average priming effects are plotted as a function of the experimental conditions.*p < .05; **p < .005. Congruent priming corresponds to the difference in response time (RT) between the incongruent prime condition (blob-like animal primes in the case of tool targets and elongated tools in the case of animal targets) and the congruent prime condition (elongated tool primes in the case of tool targets and blob-like animals in the case of animal targets). Elongated animal priming corresponds to the difference in RT between incongruent prime conditions and elongated animal primes. Finally, round “tool” priming corresponds to the difference in RT between incongruent prime conditions and round “tool” primes. Error bars represent the standard errors of the means for priming effects across participants. a Results for Experiment 1a, where continuous flash suppression was used to render primes invisible. b Results for Experiment 1b, where backward masking was used to render primes invisible
For the BM experiment (Experiment 1b; N = 29), however, only elongated tool primes (e.g., hammer) facilitated categorization responses to tool targets, when compared with the blob-like animal primes [e.g., cow; average priming for elongated tools = 9 ms, SEM = 3 ms, t(28) = 2.86, p = .008; all other ts < 1; Fig. 2b]. Elongated-shaped animal primes (e.g., fish), as well as blob-like animal primes (e.g., cow), led to faster categorization responses for animal targets, when compared with elongated tool prime pictures [e.g., hammer; average priming for blob-like animals = 16 ms, SEM = 6 ms, t(28) = 2.55, p = .017; average priming for elongated animals = 13 ms, SEM = 6 ms, t(28) = 2.06, p = .049; Fig. 2b]. Somewhat out of line with the overall pattern, blob-like “tools” (e.g., doorknob) facilitated the categorization of animal targets, but not that of tool targets [average priming for blob-like “tools” on animal targets = 10 ms, SEM = 4 ms, t(28) = 2.77, p = .01; Fig. 2b].
Discussion
In Experiment 1, we demonstrated that elongated objects, irrespective of their semantic category membership, when under situations that bias processing toward the dorsal stream (i.e., under CFS), influence the categorization of manipulable objects. In contrast, when visual processing is not biased to the dorsal visual stream (i.e., under BM), information about an object’s semantic category membership supersedes elongation and drives priming effects.
Note, however, that for Experiment 1b (i.e., under BM), blob-like “tool” primes did not affect the categorization of tool targets more than blob-like animal primes. Although these items are clearly recognizable as manipulable objects, their “toolness” may be hard to extract in situations where the signal is impoverished (e.g., BM-induced suppression). Interestingly, under the same masking condition, these primes elicited priming of animal targets, suggesting that information about the shape of these object primes was nevertheless available and served to prime an overall shape that was congruent with the target animals. Importantly, the overall pattern of results from Experiment 1b demonstrates that the priming effect elicited by elongated primes under CFS is not a reflection of their lack of “animalness” but, rather, of the differential processing underlying CFS and BM.
The interpretation of the data from Experiment 1 that we therefore favor is that elongation is a visual feature that “triggers” processing of a stimulus by the dorsal stream and activity within dorsal stream structures is able to influence a subsequent categorization of a tool. However, it is important to consider alternative explanations that collectively derive from the design choice of using only elongated tools as targets. Thus, one possible alternative explanation for our data would be that our results reflect strict form priming. Such visual form priming effects would be observed only for the conditions where elongated primes (which can be animals or tools) precede elongated targets (which, in our experiments, were only tools), but not when blob-like primes (which can be animals or tools) precede blob-like targets (which in our experiments were only animals). This would be so because these elongated-shaped primes would be preferentially available under CFS, when compared with blob-like-shaped primes. This alternative view differs from what we propose herein, in that it suggests that it is not an effect of elongation (of primes) on tool categorization, but an effect of an elongated prime on an elongated target. This issue, of whether the selectivity in the effects that we observe depends on the target tool stimuli being elongated, is important to consider. There are several directions from which it can be approached.
First, one issue that needs to be considered is whether CFS, as a technique, is less effective in suppressing elongated than blob-like shapes. To the best of our knowledge, there are no data that suggest that elongated shapes are less suppressed than blob-like shapes by CFS (see the Discussion section of Experiment 2 for additional consideration). In fact, we, and others, have shown that blob-like shapes (e.g., faces) can influence behavioral and neural responses under CFS (Almeida, Pajtas, Mahon, Nakayama, & Caramazza, 2013; Jiang & He, 2006; Pasley, Mayes, & Schultz, 2004; Yang & Blake, 2012). Specifically, an emotional face (a blob-like shape) can interfere with likability judgments over a neutral nonface item, whereas an elongated shape (a polygon) does not (Almeida et al., 2013), suggesting that elongated and blob-like shapes do not differ in their general suppressibility or accessibility under CFS.
Another direction from which an alternative explanation could be considered is whether the pattern we have observed is the result of a strictly form-based priming effect. In other words, elongated stimuli selectively prime tool targets because of similarity in visual form, and not because of a privileged relationship between elongation and the dorsal stream. However, on this account, it is not clear why CFS-suppressed blob-like primes (i.e., animal and blob-like tool primes) do not prime blob-like targets (i.e., blob-like animal targets). That is, why don’t we see form priming effects for animals under CFS? This question is ever the more pressing, on this alternative explanation, in the context of the fact that we do see priming for blob-like animal primes under BM (see also Almeida et al., 2008). Another argument against a form-based interpretation of the data from Experiment 1 is that CFS-suppressed identity primes, where the visual forms of the prime and the target are exactly the same, produce no more priming than do CFS-suppressed category congruent primes (Almeida et al., 2010).
It might be argued that a decisive test of these issues would be to repeat Experiment 1, but to use blob-like tool targets. However, even if it were the case that there was no priming of CFS-suppressed elongated tools on blob-like tool targets, that would be consistent with either of two alternatives: (1) the original effect is a form-based phenomenon, or (2) blob-like stimuli do not have a special privileged to be processed by the dorsal stream. Thus, such an experiment would not, in the end, be conclusive. Given that there is independent reason to be cautious about the contribution of form-based priming (blob-like primes do not prime blob-like targets, and no added priming from identity primes), we believe that the balance of evidence argues against a form-based account. Thus, without denying possible contributions of any form-based priming, it seems that being an elongated object is the critical dimension determining whether primes affect tool target processing when the primes are rendered invisible with CFS. We suggest that this is because elongation is the critical dimension determining whether primes affect tool target processing when these primes are rendered invisible with CFS, because their processing is biased toward the dorsal stream. Nevertheless, it is important to bear in mind that the conclusions that can be drawn from these experiments do have the limitation that they are restricted to a situation in which the target tools are elongated. That said, we sought to provide additional positive evidence for our central hypothesis using other experimental approaches to bias processing of visual stimuli toward the dorsal visual pathway.
Experiment 2
The CFS-specific results presented in Experiment 1a were further studied in a second experiment, where simple elongated or blob-like nonobject shapes (i.e., a solid rod and a solid circle; Fig. 1a) were presented as primes instead of the object pictures used previously. The use of these simple shapes, instead of real objects, allowed us to isolate the minimal features that are needed to trigger activity within the dorsal stream, which then, by hypothesis, affects categorization of manipulable objects.
Method
Participants
Eighty-seven undergraduate students participated in the study in exchange for course credit or payment. All participants had normal or corrected-to-normal vision, were right-handed, and gave written informed consent. Participants were naïve as to the experimental hypotheses. The project was approved by the appropriate institutional review board.
Materials and procedure
We used the same black-and-white target pictures as in Experiment 1. For prime stimuli, we used one obliquely oriented rod and one centrally presented circle (see Fig. 1a for the exact prime stimuli used). In this experiment, each target picture was presented 20 times, and each prime picture was presented 80 times, for a total of 160 trials.
As in Experiment 1a, the primes were rendered invisible via CFS (see Fig. 1b). After the experiment proper, participants completed a prime discrimination task that was similar to the one in Experiment 1a, except that participants judged whether they saw a rod or a circle. Only participants who performed at chance in the prime discrimination task were included in the analyses of response time (see Fig. S2c for more detailed analyses).
This experiment was run on a Dell PC, with a ViewSonic Ultrabrite A90_f monitor. The monitor refresh rate was 100 Hz. Stimuli were presented using DisplayMaster DirectX (DMDX) software (Forster & Forster, 2003). This experiment lasted approximately 10 min.
Analysis
We used planned contrasts to analyze response latencies (Rosenthal et al., 2000). For each category in each experiment, one pairwise contrast was employed over the difference in response time between incongruent trials (circle for tool targets and rod for animal targets) and the congruent condition (rod for tool targets and circle for animal targets). The analysis of prime awareness data followed the same steps as in Experiments 1a and 1b.
Results
As in the previous experiments, participants whose prime discrimination levels were not above chance were included in the main analysis (see Table 1). Out of the 87 participants, 46 were included in the main analysis, since they were objectively and subjectively unaware of the prime pictures.
Rectangular and circular shapes, rendered invisible with CFS, resulted in significant priming effects, but only for target tools (as in Experiment 1a; for mean response times, see Table 3). Participants were faster to categorize tool targets in the presence of a CFS-suppressed elongated-shaped prime (i.e., a rod) than in the presence of a blob-like-shaped prime [i.e., a circle; N = 46; average priming for the rod during categorization of tool targets = 7 ms, SEM = 3 ms; t( 45) = 2.17, p = .035; for animal targets, t < 1].
Discussion
The results from Experiment 2 demonstrate that shape elongation, when processed by the dorsal stream, and even when carried by a category-neutral stimulus, affects tool categorization but not animal categorization. Again, as was discussed after Experiment 1, this effect must be taken in the context of the fact that all of the tool targets were elongated themselves.
Experiment 3
In Experiments 1 and 2, we exploited the neural signatures of CFS and BM to bias prime processing toward the dorsal stream and showed that object elongation, when processed by the dorsal stream, affects categorization of tool targets. As was described in the Introduction, the use of CFS as a tool to dissociate processing within dorsal and ventral stream regions has been challenged (e.g., Hesselmann & Malach, 2011; Sterzer et al., 2008). Although the balance of evidence still seems to suggest that CFS can be useful in inducing biases in visual processing toward dorsal stream structures, converging data from other methods and manipulations may be needed to support the claim that object elongation, when processed by the dorsal stream, affects decisions about tool targets. It has been shown that the effectiveness of the masks traditionally used under CFS may not uniformly apply to all types of object features (Yang & Blake, 2012). In particular, Yang and Blake suggested that diagonally oriented stimuli are more prone to escape CFS suppression than are other types of stimuli. Despite the fact that our stimuli span different orientations (see Fig. S1), that the spatial distribution of the masks we used for CFS is very similar to the one used for BM, and that the strict prime awareness criteria employed would excluded participants whose signal-to-noise ratio for elongated objects was significantly higher than for blob-like objects, it might be maintained that the potential inefficiency of CFS in suppressing elongated objects may have contributed to the results of these two experiments. Finally, CFS and BM have very different time courses, and these timing signatures may interact differentially with the effects observed for elongated and blob-like objects.
To address these lingering issues and to further show that elongation, when processed by dorsal stream structures, can be used to inform manipulable object categorization, we used BM in Experiments 3 and 4 and exploited other psychophysical dimensions that are thought to bias processing toward the dorsal visual stream. These two experiments are crucial since they circumvent the issues that may be raised for the argument that CFS biases analysis toward the dorsal stream, while permitting other means for testing the theoretical prediction that the visual dimension of elongation triggers analysis by the dorsal stream and, hence, has a privileged relationship to conceptual decisions about tools.
In Experiment 3, we explored the fact that the neural networks that process tools within the dorsal and ventral streams have different degrees of lateralization. Neural specificity for tools tends to be bilateral in ventral stream structures but left-lateralized in parietal and frontal regions (for right-handers; Chao & Martin, 2000; Handy et al., 2003; Johnson-Frey et al., 2005; Mahon et al., 2007). As such, lateralized presentations of backward-masked primes should result in different sensitivities to tool-related dorsal stream processing for primes presented in the RVF and in the LVF (Garcea et al., 2012). Specifically, we predicted that backward-masked elongated animal primes would prime tool targets when presented in the RVF, but not in the LVF, whereas tool primes should facilitate the categorization of tool targets regardless of the side on which the tool primes were presented.
Method
Participants
Fifty-three undergraduate students participated in the study in exchange for course credit. All participants had normal or corrected-to-normal vision, were right-handed, and gave oral informed consent. Participants were naïve as to the experimental hypotheses. The project was approved by the appropriate institutional review board.
Materials and procedure
We used the same black-and-white target and prime pictures as in Experiment 1, with the exception of the blob-like tool primes. This condition was dropped from the experiment, since we focused on the condition of interest—elongated animals. Each target picture appeared 72 times, and each prime picture was presented 48 times, for a total of 576 trials. The procedure followed the one used in Experiment 1b, except that backward-masked primes were not presented in the center of the screen. For half of the trials, prime pictures were presented in the RVF, whereas on the other half, they were presented in the LVF. The border of the prime pictures that was closest to fixation was positioned 3.5° of visual angle away from fixation. Immediately after the prime pictures, and irrespective of the prime location, two masks were presented, one on each side, spatially overlapping the possible locations of the primes (see Garcea et al., 2012). Immediately after the masks, a centrally presented target appeared and remained on the screen for 3 s or until a response was made, whichever came first. After the experiment proper, participants completed a prime discrimination task similar to those in previous experiments, except that the primes were again presented in the RVF or LVF. Only participants who performed at chance level in the prime discrimination task were included in the main analyses reported here (see Fig. S2d for more detailed analyses).
Experiments were run on a PC, with a Samsung SyncMaster 793DF monitor. The monitor refresh rate was 75 Hz. Stimuli were presented using MATLAB and the Psychophysics Toolbox extensions (e.g., Brainard, 1997). This experiment lasted approximately 1 h.
Analysis
Planned contrasts were used to analyze response latencies (Rosenthal et al., 2000). For each category and prime location, two pairwise contrasts were employed to test for priming effects in categorization response times. The priming effect was calculated as the difference in response time between incongruent trials and the other conditions. For animal targets, elongated tool primes were considered the canonical incongruent condition and were contrasted with blob-like animal and elongated animal prime pictures. For tool targets, blob-like animal primes were considered as the canonical incongruent prime condition and were compared with elongated tool and elongated animal prime pictures. The analysis of prime awareness data followed the same steps as in Experiments 1a and 1b.
Results
Data from the independent prime awareness task were used as a criterion to include participants in the main analysis (see Table 1). Out of the 52 participants, 42 were included because they were subjectively and/or objectively unaware of the prime pictures during the experiment.
The analysis of categorization times showed that tool primes, when compared with blob-like animal primes, facilitated the categorization of tool targets irrespective of whether the primes were presented in the RVF or the LVF [average priming effects for tool primes in the RVF = 23 ms, SEM = 5 ms, t(41) = 4.84, p < .0001; average priming effects for tool primes in the LVF = 11 ms, SEM = 4 ms, t(41) = 2.685, p = .011; see Fig. 3; for mean response times, see Table 4]. More relevant for our present hypothesis is the analysis of whether elongated animals, when compared to blob-like animal primes, affect the categorization of tool targets. This analysis yielded differential results depending on the side of prime presentation. Specifically, elongated animal primes presented in the RVF, when compared with RVF-presented blob-like animal primes, led to faster categorization times for tool targets [average priming effects for elongated animal primes in the RVF = 13 ms, SEM = 5 ms; t(41) = 2. 854, p = .017]. In contrast, when the same comparison was made for the elongated and blob-like animal primes presented in the LVF, no significant difference was obtained (average priming effects for elongated animal primes in the LVF = 4 ms, SEM = 5 ms; t < 1). As was predicted, while congruent priming effects for tool targets were obtained for RVF and LVF prime presentations, the effect of elongated animal primes over tool targets was appreciable only when the primes were presented in the RVF.
Behavioral priming effects for Experiment 3. Average priming effects are plotted as a function of the experimental conditions and side of presentation.*p < .05; **p < .005. Congruent priming corresponds to the difference in response time (RT) between the incongruent prime condition (blob-like animal primes in the case of tool targets and elongated tools in the case of animal targets) and the congruent prime condition (elongated tool primes in the case of tool targets and blob-like animals in the case of animal targets). Elongated animal priming corresponds to the difference in RT between incongruent prime conditions and elongated animal primes. Error bars represent the standard errors of the means for priming effects across participants
Categorization times for animal targets revealed priming effects that were not equally distributed across the LVF and RVF. Animal primes presented in the RVF, irrespective of whether they were elongated or not, facilitated the categorization of animal targets [average priming effects for blob-like animal primes in the RVF = 22 ms, SEM = 4 ms, t(41) = 3.757, p = .001; average priming effects for elongated animal primes in the RVF = 13 ms, SEM = 5 ms, t(41) = 2.878, p = .006; see Fig. 3]. Animal primes presented in the LVF unexpectedly did not facilitate animal target categorization [average priming effects for blob-like animal primes in the LVF = 6 ms, SEM = 6 ms, t(41) < 1; average priming effects for elongated animal primes in the LVF = 6 ms, SEM = 4 ms, t(41) = 1.409, p = .166; see Fig. 3].
Discussion
The results of Experiment 3 show that in experimental conditions where participants are categorizing tool targets and where the processing of prime stimuli is putatively biased toward the dorsal stream (i.e., RVF presentations), elongated objects, irrespective of their semantic category membership, facilitate the processing of tool targets. These results are particularly striking because RVF and LVF prime conditions differ only in the locus of presentation of the prime picture. Our results also show an unexpected imbalance in the priming effects observed for animal target pictures. Although beyond the scope of the present investigation, there are reports that show that priming effects for primes presented in the LVF are weak or nonexistent (e.g., Abernethy & Coney, 1993; Koivisto & Revonsuo, 2000; Lovseth & Atchley, 2010).
Experiment 4
In Experiment 4, in conjunction with BM, we used a dependent measure that is more sensitive to effects emerging from dorsal stream structures—reaching trajectories. In this experiment, we asked participants to reach and touch one of two spots on the screen. Because reaching and pointing are heavily dependent on online visuomotor corrections and on the processing subserved by regions within the dorsal stream (e.g., Batista et al., 1999; Connolly et al., 2003), we predicted that hand trajectories would show processing similarities for tool and elongated animal primes, especially when compared with blob-like animals, that were not apparent in Experiment 1b. That is, given the reliance of reaching on dorsal stream structures, the approach of Experiment 4 allowed for effects of dorsal stream processing to be expressed in participants’ categorization decisions with BM-suppressed primes. Thus, Experiment 4 presented primes and targets in exactly the same manner as in Experiment 1b but used reaching trajectories instead of response latencies as the dependent measure. This is not to say that reaching trajectories would express preferentially effects mediated by the dorsal stream in an overall and absolute fashion but, rather, that, as compared with the dependent variable used in Experiment 1b (i.e., buttonpresses), reaching trajectories might be more sensitive in expressing dorsal stream processing.
Method
Participants
Fifty-eight students participated in the study in exchange for course credit. All participants had normal or corrected-to-normal vision, were right-handed, and gave oral informed consent. Participants were naïve as to the experimental hypotheses. The project was approved by the appropriate institutional review board.
Materials and procedure
In this experiment, we used the same materials as in Experiment 3 and the same procedures as in Experiment 1b, with the exception that the dependent measure was reaching movements instead of buttonpresses. The trial structure used in this experiment was exactly the same as in Experiment 1b, with a central prime followed by a mask that was immediately followed by a target picture. On each trial, participants were instructed to first press a button that would trigger the initiation of that trial. The button was aligned with the participant’s midline and was positioned 5 cm way from her/his body. Immediately upon presentation of the target, participants initiated a reaching movement as quickly as possible. Their task was to touch a square presented on either the right or the left border of the monitor, according to the category of the target. Trials where movement initiation was slower than 350 ms or initiated before the target picture was presented were aborted and repeated at the end of the blocks. Participants received training before the experiment proper started. The mapping between category of the target and side of response was counterbalanced across participants. After the experiment proper, participants completed a prime discrimination task that was similar to the one in Experiment 1b and where the responses were collected through buttonpresses. Only participants who performed at chance level in the prime discrimination task were included in the main analyses reported here (see Fig. S2e for more detailed analyses).
All experiments were run on a PC, with a Samsung SyncMaster 793DF monitor. The monitor refresh rate was 75 Hz. Stimuli were presented using MATLAB and the Psychophysics Toolbox extensions (e.g., Brainard, 1997). An Optotrak 3D Investigator™ Motion Capture System from Northern Digital Inc. sampling 3-D coordinates at 200 Hz was used to record the reaching movements. This experiment lasted approximately 40 min.
Analysis
For the analysis of motion-tracking data, we followed standard procedures (e.g., Finkbeiner & Friedman, 2011). From the pointing data, we extracted a dependent measure that related to prime-specific deviation in reach trajectories. We first calculated, for each trajectory, the straight line that united the trajectory’s start and end points (i.e., an optimal trajectory). We then calculated, per trajectory, the area between this straight line and the actual trajectory—that is, the area under the curve for each trajectory. The larger the area under the curve, the stronger the interference effect of the prime on that particular trajectory; congruent primes should lead to smaller areas under the curve than incongruent primes.
Planned contrasts were then used over the mean areas under the curve for each condition (Rosenthal et al., 2000). For each category, we contrasted the area under the curve for the incongruent prime conditions with those for the other two conditions. For animal targets, elongated tool primes were considered as the canonical incongruent condition and were contrasted against blob-like animal and elongated animal prime pictures. For tool targets, blob-like animal primes were considered as the canonical incongruent prime condition and were compared with elongated tool and elongated animal prime pictures. The analysis of prime awareness data followed the same steps as in the previous experiments.
Results
Data from the prime awareness task led to the selection of 37 participants who were objectively and subjectively unaware of the prime pictures throughout the experiment.
Values for the area under the curve for all the trajectories from these 37 participants were entered into the main analysis and were used as a proxy for the deviation of a trajectory relative to an optimal trajectory. This analysis revealed that reaching trajectories varied across experimental conditions (see Fig. 4). There was a trend for trajectories in response to tool targets to deviate more when those targets were preceded by blob-like animal primes than when preceded by tool primes [average difference between the area under the curve = 2.3 mm2, SEM = 1.4 mm2; t(36) = 2.334, p = .096] and a clear effect comparing blob-like animals and elongated animals [average difference between the area under the curve = 2.7 mm2, SEM = 0.9 mm2; t(36) = 2.711, p = .0031; see Fig. 5]. These effects indicate that, for tool targets, elongated animal primes are processed similarly to tool primes, despite their semantic category, and lead to diminished shifts in trajectory, when compared with blob-like animal prime pictures.
Sample data from hand trajectories for Experiment 4. a All hand trajectories for a representative participant. b Average hand trajectories (solid lines) and 2.5 standard deviation (dashed lines) for a representative participant, by condition. c Average hand trajectories by condition for all participants (results normalized to the animal-left/tool-right response mapping)
Behavioral priming effects for Experiment 4: average values for the areas under the curve plotted as a function of the experimental conditions. ¥ p = .096; *p ≤ .05; **p < .005. Error bars represent standard errors of the means for priming effects across participants
Trajectories in response to animal targets were also affected by the prime pictures. Tool primes, when compared with both types of animal primes, led to more appreciable deviations in hand trajectories, as measured by larger areas under the curve for the tool prime condition [average difference between the area under the curve for tool and blob-like animal primes = 3 mm2, SEM = 1.3 mm2, t(36) = 2.252, p = .031; average difference between the area under the curve for tool and elongated animal primes = 2.4 mm2, SEM = 1.2 mm2, t(36) = 2.401, p = .053; see Fig. 5].
Discussion
In Experiment 4, we used a dependent measure—reaching trajectories—that is more sensitive to effects originating in the dorsal stream than are traditional dependent measures, such as the one used in Experiment 1b. In contrast to the results obtained in Experiment 1b, the results of Experiment 4 show that elongated animal and tool primes lead to shifts in reach trajectories that are less pronounced than those observed from animal primes when tool targets are categorized. That is, this dependent measure revealed that object elongation can affect tool recognition, again, at least for elongated tool targets. On the other hand and in line with the results of Experiment 1b, shifts in reach trajectory were more pronounced for tool primes than for both kinds of animal primes, when animal targets were categorized.
Interestingly, our data do not show any advantage for a tool prime, when compared with an elongated animal prime, for the categorization of tool targets. It could have been speculated that because tool primes not only are elongated, but also belong to the same semantic category as the targets, these primes should lead to trajectories that are even closer to the optimal direct trajectory than do elongated animal primes. It may be the case that trajectory deviations for the processing of tool and animal targets are differentially dependent on information that is available in different temporal windows. Information on object elongation may be available earlier than categorical information, due to faster processing in dorsal stream regions. As such, when information about the category of an elongated animal prime picture becomes available, the trajectory is already being guided by the notion that there is an elongated object, which will, in turn, signal the presence of a graspable object. This information about graspability is then confirmed by the (putatively) slower but detailed analysis of the input by the ventral visual pathways.
General discussion
Recently, it has been shown that dorsal stream information affects conceptual decisions about manipulable objects (Almeida et al., 2010; Almeida et al., 2008; Helbig et al., 2006). Here, in a series of experiments, we addressed the nature of this information. In Experiments 1a and 2, primes were presented under CFS, a technique that biases processing toward the dorsal visual stream; those primes consistently influenced manipulable object categorization if they had an elongated shape, irrespective of their semantic category membership. Experiment 1b extended these results by showing that when prime pictures are processed by a more extended set of brain regions including the ventral stream (using BM to render the primes invisible), semantic category membership information supersedes the effect of elongation. In Experiment 3, backward-masked elongated primes presented in the RVF, which should, by hypothesis, have privileged access to the left-lateralized dorsal stream tool-specific network, facilitated processing of tool targets. Finally, in Experiment 4, we used a dependent measure that relied more on dorsal stream processing and showed that deviations in reach trajectories toward tool targets were stronger for blob-like animal primes than for elongated animal or tool primes.
By using these different procedures and psychophysical manipulations to address the role of dorsal stream information in manipulable object recognition, we were able to capitalize on the relative strengths of the different approaches and show that the basic phenomenon is invariant to various weaknesses that may be attend any given paradigm. These results demonstrate that (1) a visuomotor perceptual feature—object elongation—triggers analysis by the dorsal visual pathway and (2) when the dorsal visual pathway is engaged by a stimulus, conceptual decisions about subsequently presented tool stimuli are also modulated.
What is it about elongated objects, or the feature “elongation,” that leads to these phenomena? One response is that effects occur over conceptual information: Elongation invites grasp preparation, and the preparation of the grasping system (even diffusely or generally) leads to activation spreading to object concepts that we commonly grasp (e.g., Almeida et al., 2010; Almeida et al., 2008; Culham et al., 2006). Another possibility is that it is the priming of grasp information itself that is relevant and, in the course of making conceptual decisions, the cognitive system takes into account the current state of the sensorimotor system (Mahon & Caramazza, 2005). In other words, grasping, like many other kinds of actions, is, in part, dependent on sensory information that is inherently uncertain. Any dimension that helps disambiguate environmental uncertainty will lead to a faster and more robust definition of the graspable status of a target object, and the graspable status of an object is relevant to conceptual processing.
Elongation may be one such dimension. A rod-like surface reduces the degrees of freedom within the motor system, since optimal grasp points will be along the longitudinal axis of the object, provided that the rod is not extremely thin and the longitudinal axis is sufficiently wide; grasp points will also probably be located in or around the center of mass of the object (e.g., Blake, 1992; Iberall, Bingham, & Arbib, 1986; Lederman & Wing, 2003). In fact, when the grasp points are not at the center of mass, a torque will be created, and grasp equilibrium will not be as easily met (e.g., Iberall et al., 1986; Lederman & Wing, 2003). For a blob-like object (e.g., a circle), any diameter passes through the center of mass (assuming an even distribution of weight throughout the object), and as such, the selection of the particular grasp points to be used is dependent on information other than that immediately available from visual inspection. On the other hand, the selection of grasp points for an elongated object (e.g., an ellipse or rectangle) is much more constrained by the geometrical properties of the object and the location of its center of mass (Lederman & Wing, 2003), since there is a limited set of grasp points that fall on the object’s center of mass. In fact, when one interacts with an object, an object’s projection profile increases the probability of shaping one’s hand to perform a grasp (Klatzky, McCloskey, Doherty, Pellegrino, & Smith, 1987). Moreover, such an elongated shape immediately provides a graspable surface—a handle. Therefore, the processing of elongated objects within the dorsal stream may prompt a limited set of visuomotor descriptions, or even a unique description, to guide motor interaction. This unambiguous description grants the system more independence from inputs from other brain regions to select and prepare the most appropriate grasp. We propose that it is this enhanced grasping status, and visuomotor preparation, that may be useful in assisting conceptual decisions about manipulable objects. However, it is important to note, on such an account, that object grasping and object concepts are established to doubly dissociate under conditions of brain damage.
These data and considerations converge with observations from the classic agnosic patient D.F.—who presented with bilateral lesions to her ventral stream—and with the performance of optic ataxic patients, who typically present with lesions within dorsal stream regions. Goodale, Meenan, et al. (1994) showed that D.F. grasped simple objects, with a unique or unambiguous principal axis, near or around the center of mass, similarly to healthy controls. In contrast, optic ataxic patient R.V. positioned her fingers much further away from the center of mass and, thus, failed to grasp the objects optimally. Interestingly, when patient D.F. is presented with objects that lack a unique or unambiguous elongated principal axis (e.g., T-shaped or cross-shaped objects) and, therefore, there are competing visuomotor descriptions available, her dorsal stream is no longer capable of dictating the proper commands for flawless action performance (Carey, Harvey, & Milner, 1996; Goodale, Jakobson, Milner, & Perrett, 1994). In such situations, the preparation and execution of the grasp itself is well formed as a visuomotor act, but it is not directed toward parts of the object that then facilitate goal-directed behavior toward the object. This is (presumably) because the dorsal stream, in and of itself, is not able to select among different grasps that are all optimal from a strict visuomotor perspective but only one of which is optimal with respect to the goals of the action. However, other theoretical possibilities exist (for a discussion, see Mahon & Wu, in press).
It must be noted, however, that processing within the dorsal stream is much richer than what we have been proposing up to now. For instance, there are many neuroimaging reports that implicate dorsal stream structures in the preparation and comprehension of tool manipulation (Boronat et al., 2005; Johnson-Frey et al., 2005; Kellenbach, Brett, & Patterson, 2003). Moreover, research on apraxia has shown that some of these dorsal stream structures are causally implicated in the manipulation of objects (Buxbaum, Kyle, Grossman, & Coslett, 2007; Goldenberg & Spatt, 2009; Haaland, Harrington, & Knight, 2000; Sirigu, Duhamel, & Poncet, 1991). Clearly, tool-related dorsal stream processing is not exhausted by the processing of an object’s axis of elongation. Notwithstanding, we believe that the structures within the dorsal stream that are least dependent on input from elsewhere—namely, the ventral stream—may be limited in their capacity to process higher-level tool-related information and are, hence, restricted to a strict visuomotor analysis of the surrounding environment. In such situations, object elongation (and perhaps other visuomotor dimensions, such as size and orientation) can be very useful in defining the graspable status of an object.
Recently, we have used functional MRI to show that tool processing within superior (and posterior) parietal regions is less dependent on input from ventral temporal regions (in comparison with inferior parietal regions; Almeida, Fintzi, & Mahon, 2013; Mahon, Kumar, & Almeida, 2013). Specifically, psychophysical manipulations of stimuli that bias processing toward the dorsal stream lead to selective activation for tool images in superior and posterior parietal regions, in the vicinity of the activations reported by Fang and He (2005) when tool stimuli were rendered invisible during fMRI with CFS. More generally, the location of these superior and posterior parietal regions aligns well with the set of parietal regions that are typically damaged in patients with impairments for reaching and grasping (i.e., optic ataxic deficits; e.g., Perenin & Vighetto, 1988; see also Culham et al., 2003, for convergence fMRI data). Additional convergence evidence is provided by the results of Sakata and colleagues (e.g., Sakata et al., 1998; see also Shikata et al., 2001), who found that the firing rate of a population of neurons in the caudal intraparietal sulcus increases monotonically with increasing length of the stimulus and decreases with increasing thickness of the elongated stimuli. Finally, these more posterior parietal regions seem to be involved in the processing of other visuomotor dimensions, such as object orientation and, perhaps, size, in support of object grasping (e.g., James, Humphrey, Gati, Menon, & Goodale, 2002). Whether there are similar visuomotor effects of orientation or size—and possibly, other dimensions—under experimental conditions that bias analysis toward the dorsal stream should be explored in future experiments.
As was noted above (see the Discussion section of Experiment 1), the conclusions that are afforded by this series of experiments are constrained by the fact that the tool targets were always themselves elongated. Although the weight of empirical evidence argues against an account in terms of strict visual form priming, it could be that our effects were due to the fact that elongated animals primes and tool targets shared the elongated profile. This alternative view is not dramatically different from our perspective. We believe, however, that it is not form per se that drives our effects but, rather, what can be extracted from the processing of an elongated object within the dorsal stream that then affects the categorization of elongated tool targets. Nevertheless, because we have not addressed this issue directly and empirically, further studies will be needed to fully understand the role of object elongation in driving the phenomena that we have reported.
Our results suggest that locally within the dorsal stream, there is limited, if any, conceptual processing of objects. Without reliable or relevant input from elsewhere in the brain (e.g., identity or categorical information processed by regions within the ventral stream or the prefrontal cortex), the dorsal stream structures (perhaps those that are more posterior) are restricted to strict visuomotor shape-dependent information. Under such processing situations, what these dorsal stream structures care about is whether a stimulus is graspable and the ease and robustness with which it can establish that. Importantly, we show that this kind of information can be relevant to conceptually based decisions about manipulable objects. When the category of a manipulable object is determined, the understanding that a stimulus is graspable may be sufficiently diagnostic to affect the categorization decision. Interestingly, however, this information can be superseded by the category information about an item, when available, suggesting promiscuity within the cognitive system. Specifically, according to the use demanded of the information by a particular task, different types of information will be accentuated in the service of fulfilling the current task goals.
References
Abernethy, M., & Coney, J. (1993). Associative priming in the cerebral hemispheres as a function of SOA. Neuropsychologia, 31, 1397–1409.
Almeida, J., Fintzi, A., & Mahon, B.Z. (2013). Tool Manipulation Knowledge is Retrieved by way of the Ventral Visual Object Processing Pathway. Cortex, 10.1016/j.cortex.2013.05.004 DOI:10.1016/j.cortex.2013.05.004#doilink
Almeida, J., Mahon, B. Z., & Caramazza, A. (2010). The role of the dorsal visual processing stream in tool identification. Psychological Science, 21(6), 772–778.
Almeida, J., Mahon, B. Z., Nakayama, K., & Caramazza, A. (2008). Unconscious processing dissociates along categorical lines. Proceedings of the National Academy of Sciences of the United States of America, 105(39), 15214–15218.
Almeida, J., Pajtas, P. E., Mahon, B. Z., Nakayama, K., & Caramazza, A. (2013). Affect of the unconscious: Visually suppressed angry faces modulate our decisions. Cognitive, Affective, and Behavioral Neuroscience, 13, 94–101.
Batista, A. P., Buneo, C. A., Snyder, L. H., & Andersen, R. A. (1999). Reach plans in eye-centered coordinates. Science, 285, 257–260.
Blake, A. (1992). Computational modelling of hand-eye coordination. Philosophical Transactions of the Royal Society: Biological Sciences, 337(1281), 351–360.
Boronat, C. B., Buxbaum, L. J., Coslett, H. B., Tang, K., Saffran, E. M., Kimberg, D. Y., et al. (2005). Distinctions between manipulation and function knowledge of objects: Evidence from functional magnetic resonance imaging. Cognitive Brain Research, 23(2–3), 361–373.
Brainard, D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436.
Breitmeyer, B. G., & Ogmen, H. (2000). Recent models and findings in visual backward masking: A comparison, review, and update. Perception & Psychophysics, 62(8), 1572–1595.
Bub, D. N., & Lewine, J. (1988). Different modes of word recognition in the left and right visual fields. Brain and Language, 33, 161–188.
Buxbaum, L. J., Kyle, K. M., Grossman, M., & Coslett, H. B. (2007). Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex, 43(3), 411–23.
Cant, J. S., & Goodale, M. A. (2007). Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cerebral Cortex, 17, 713–731.
Carey, D. P., Harvey, M., & Milner, A. D. (1996). Visuomotor sensitivity for shape and orientation in a patient with visual form agnosia. Neuropsychologia, 34(5), 329–337.
Chao, L., & Martin, A. (2000). Representation of manipulable man-made objects in the dorsal stream. NeuroImage, 12, 478–484.
Chiarello, C., Nuding, S., & Pollock, A. (1988). Lexical decision and naming asymmetries: Influence of response selection and response bias. Brain and Language, 34, 302–314.
Connolly, J. D., Andersen, R. A., & Goodale, M. A. (2003). FMRI evidence for a “parietal reach region” in the human brain. Experimental Brain Research, 153, 140–145.
Culham, J. C., Cavina-Pratesi, C., & Singhal, A. (2006). The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia, 44(13), 2668–84.
Culham, J. C., Danckert, S., Souza, J. X. D., Gati, J., Menon, R., & Goodale, M. A. (2003). Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Experimental Brain Research, 153(2), 180–189.
Damasio, H., Tranel, D., Grabowski, T., Adolphs, R., & Damasio, A. (2004). Neural systems behind word and concept retrieval. Cognition, 92, 179–229.
Dehaene, S., Naccache, L., Cohen, L., Le Bihan, D., Mangin, J.-F., Poline, J.-B., & Rivière, D. (2001). Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience, 4(7), 752–758.
Desmurget, M., Epstein, C. M., Turner, R. S., Prablanc, C., Alexander, G. E., & Grafton, S. T. (1999). Role of the posterior parietal cortex in updating reaching movements to a visual target. Nature Neuroscience, 2, 563–567.
Fang, F., & He, S. (2005). Cortical responses to invisible objects in the human dorsal and ventral pathways. Nature Neuroscience, 8(10), 1380–1385.
Finkbeiner, M., Almeida, J., & Caramazza, A. (2006). Letter identification processes in reading: Distractor interference reveals a left-lateralized domain-specific mechanism. Cognitive Neuropsychology, 23, 1083–1103.
Finkbeiner, M., & Friedman, J. (2011). The flexibility of nonconsciously deployed cognitive processes: Evidence from masked congruence priming. PLoS ONE, 6(2), e17095. doi:10.1371/journal.pone.0017095
Forster, K. I., & Forster, J. C. (2003). DMDX: A Windows display program with millisecond accuracy. Behavior Research Methods, Instruments, & Computers, 35(1), 116–124.
Garcea, F. E., Almeida, J., & Mahon, B. Z. (2012). A right visual field advantage for visual processing of manipulable objects. Cognitive, Affective, and Behavioral Neuroscience, 12(4), 813–25.
Goldenberg, G., & Spatt, J. (2009). The neural basis of tool use. Brain, 132(6), 1645–1655.
Goodale, M. A., Jakobson, L. S., Milner, A. D., & Perrett, D. I. (1994a). The nature and limits of orientation and pattern processing supporting visuomotor control in a visual form agnosic. Journal of Cognitive Neuroscience, 6(1), 46–56.
Goodale, M. A., Meenan, J. P., Bülthoff, H. H., Nicolle, D. A., Murphy, K. J., & Racicot, C. I. (1994b). Separate visual pathways for the visual analysis of object shape in perception and prehension. Current Biology, 4, 604–610.
Goodale, M. A., & Milner, A. D. (1992). Separate visual pathways for perception and action. Trends in Neurosciences, 15(1), 20–25.
Goodale, M. A., Pelisson, D., & Prablanc, C. (1986). Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature, 320(6064), 748–50.
Grill-Spector, K., Kourtzi, Z., & Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41(10–11), 1409–1422.
Haaland, K. Y., Harrington, D. L., & Knight, R. T. (2000). Neural representations of skilled movement. Brain, 123, 2306–2313.
Handy, T. C., Grafton, S. T., Shroff, N. M., Ketay, S., & Gazzaniga, M. S. (2003). Graspable objects grab attention when the potential for action is recognized. Nature Neuroscience, 6, 421–427.
Helbig, H. B., Graf, M., & Kiefer, M. (2006). The role of action representations in visual object recognition. Experimental Brain Research, 174(2), 221–228.
Hesselmann, G., & Malach, R. (2011). The link between fMRI-BOLD activation and perceptual awareness is 'stream-invariant' in the human visual system. Cerebral Cortex, 21(12), 2829–37.
Hunter, Z. R., & Brysbaert, M. (2008). Visual half-field experiments are a good measure of cerebral language dominance if used properly: Evidence from fMRI. Neuropsychologia, 46, 316–325.
Iberall, T., Bingham, G., & Arbib, M. A. (1986). Opposition Space as a Structuring Concept for the Analysis of Skilled Hand Movements. In H. Heuer & C. Fromm (Eds.), Generation and Modulation of Action Patterns (pp. 158–173). Berlin: Springer-Verlag.
James, T. W., Humphrey, G. K., Gati, J. S., Menon, R. S., & Goodale, M. A. (2002). Differential effects of viewpoint on object-driven activation in dorsal and ventral streams. Neuron, 35(4), 793–801.
Jiang, Y., & He, S. (2006). Cortical responses to invisible faces: Dissociating subsystems for facial-information processing. Current Biology, 16, 2023–2029.
Johnson-Frey, S. H. (2004). The neural bases of complex tool use in humans. Trends in Cognitive Sciences, 8(2), 71–78.
Johnson-Frey, S., Newman-Norland, R., & Grafton, S. (2005). A distributed left hemisphere network active during planning of everyday tool use skills. Cerebral Cortex, 15, 681–695.
Kellenbach, M. L., Brett, M., & Patterson, K. (2003). Actions speak louder than functions: The importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience, 15, 20–46.
Klatzky, R. L., McCloskey, B., Doherty, S., Pellegrino, J., & Smith, T. (1987). Knowledge about hand shaping and knowledge about objects. Journal of Motor Behavior, 19, 187–213.
Koivisto, M., & Revonsuo, A. (2000). Semantic priming by pictures and words in the cerebral hemispheres. Cognitive Brain Research, 10, 91–98.
Lederman, S. J., & Wing, A. M. (2003). Perceptual judgement, grasp point selection and object symmetry. Experimental Brain Research, 152, 156–165.
Lewis, J. W. (2006). Cortical networks related to human use of tools. The Neuroscientist, 12(3), 211–231.
Logothetis, N. K., & Schall, J. D. (1989). Neuronal correlates of subjective visual perception. Science, 245(4919), 761–763.
Lovseth, K., & Atchley, R. A. (2010). Examining lateralized semantic access using pictures. Brain & Cognition, 2, 202–209.
Mahon, B. Z., & Caramazza, A. (2005). The orchestration of the sensory-motor systems: Clues from neuropsychology. Cognitive Neuropsychology, 22(3), 480–494.
Mahon, B., Kumar, N., & Almeida, J. (2013). Spatial frequency tuning reveals visuomotor interactions between the dorsal and ventral visual systems. Journal of Cognitive Neuroscience, 25(6), 862–871. doi:10.1162/jocn_a_00370
Mahon, B. Z., Milleville, S., Negri, G. A. L., Rumiati, R. I., Caramazza, A., & Martin, A. (2007). Action-related properties of objects shape object representations in the ventral stream. Neuron, 55(3), 507–520.
Mahon, B. Z., & Wu, W. (in press). Cognitive Penetration of the Dorsal Visual Stream? In J. Zeimbekis & A. Raftopoulos (Eds.), Cognitive Penetration. Oxford University Press
Martin, A. (2007). The representation of object concepts in the brain. Annual Review of Psychology, 58, 25–45.
Miceli, G., Fouch, E., Capasso, R., Shelton, J. R., Tamaiuolo, F., & Caramazza, A. (2001). The dissociation of color from form and function knowledge. Nature Neuroscience, 4(6), 662–667.
Miller, E. K., Nieder, A., Freedman, D. J., & Wallis, J. D. (2003). Neural correlates of categories and concepts. Current Opinion in Neurobiology, 13(2), 198–203.
Murata, A., Gallese, V., Luppino, G., Kaseda, M., & Sakata, H. (2000). Selectivity for the shape, size and orientation of objects for grasping in neurons of monkey parietal area AIP. Journal of Neurophysiology, 83(5), 2580–2601.
Noppeney, U., Price, C., Penny, W., & Friston, K. (2006). Two distinct neural mechanisms for category selective responses. Cerebral Cortex, 16(3), 437–445.
Pasley, B. N., Mayes, L. C., & Schultz, R. T. (2004). Subcortical discrimination of unperceived objects during binocular rivalry. Neuron, 42, 163–172.
Perenin, M. T., & Vighetto, A. (1988). Optic ataxia: a specific disruption in visuomotor mechanisms i. Different aspects of the deficit in reaching for objects. Brain, 111(3), 643–674.
Prado, J., Clavagnier, S., Otzenberger, H., Scheiber, C., Kennedy, H., & Perenin, M. T. (2005). Two cortical systems for reaching in central and peripheral vision. Neuron, 48(5), 849–58.
Rolls, E. T., & Tovee, M. J. (1994). Processing speed in the cerebral-cortex and the neurophysiology of visual masking. Proceedings of the Royal Society B: Biological Sciences, 257(1348), 9–15.
Rosenthal, R., Rosnow, R. L., & Rubin, D. B. (2000). Contrasts and effect sizes in behavioral research: A correlational approach. Cambridge, England: Cambridge University Press.
Sakata, H., Taira, M., Kusunoki, M., Murata, A., Tanaka, Y., & Tsutsui, K. (1998). Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Philosophical Transactions of the Royal Society B: Biological Sciences, 353(1373), 1363–1373.
Shikata, E., Hamzei, F., Glauche, V., Knab, R., Dettmers, C., Weiller, C., & Buchel, C. (2001). Surface orientation discrimination activates caudal and anterior intraparietal sulcus in humans: An event-related fMRI study. Journal of Neurophysiology, 85, 1309–1314.
Sirigu, A., Duhamel, J. R., & Poncet, M. (1991). The role of sensorimotor experience in object recognition. Brain, 114, 2555–2573.
Song, J., & Nakayama, K. (2008). Numeric comparison in a visually-guided manual reaching task. Cognition, 106, 994–1003.
Sterzer, P., Haynes, J. D., & Rees, G. (2008). Fine-scale activity patterns within high-level ventral visual areas encode the category of invisible objects. Journal of Vision, 8(15), 1–12.
Tong, F., Nakayama, K., Vaughan, J. T., & Kanwisher, N. (1998). Binocular rivalry and visual awareness in human extrastriate cortex. Neuron, 21, 753–759.
Tranel, D., Damasio, H., & Damasio, A. R. (1997). A neural basis for the retrieval of conceptual knowledge. Neuropsychologia, 35, 1319–1327.
Tsuchiya, N., & Koch, C. (2005). Continuous flash suppression reduces negative afterimages. Nature Neuroscience, 8(8), 1096–1101.
Yang, E., & Blake, R. (2012). Deconstructing continuous flash suppression. Journal of Vision, 12(3), 1–14.
Acknowledgments
We thank M. Clara Barata and Thomas McKeeff for their comments on earlier versions of the manuscript and Matthew Finkbeiner and Jason Friedman for their help with the analysis of reach trajectories. J.A. was supported by funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement PCOFUND-GA-2009-246542 and from the Foundation for Science and Technology of Portugal. J.A., A.D., and J.F.M. were sponsored by a Foundation for Science and Technology of Portugal Project Grant PTDC/PSI-PCO/114822/2009. A.C. was supported by National Institute on Deafness and Other Communication Disorders Grant R01 DC006842 and by the Fondazione Cassa di Risparmio di Trento e Rovereto. B.Z.M. was supported in part by R21 NS076176 from NINDS. This research was supported by Foundation for Science and Technology of Portugal Project Grant PTDC/PSI-PCO/114822/2009.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1545 kb)
Rights and permissions
About this article
Cite this article
Almeida, J., Mahon, B.Z., Zapater-Raberov, V. et al. Grasping with the eyes: The role of elongation in visual recognition of manipulable objects. Cogn Affect Behav Neurosci 14, 319–335 (2014). https://doi.org/10.3758/s13415-013-0208-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0208-0