Abstract
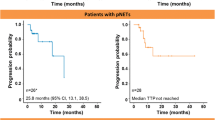
The aim of this retrospective study was to evaluate the efficacy, safety, and tolerability of lanreotide autogel given to metastatic well-differentiated (WD) neuroendocrine tumors (NET) patients observed in our Institute between 2005 and 2008. Patients with metastatic NET referred to our tertiary referral center were given lanreotide autogel 120 mg/month by deep sc injection for a period of at least 24 months. The efficacy was evaluated by the relief of disease symptoms, behavior of tumor markers and response rate in terms of time to tumor progression. Safety and tolerability were evaluated by assessing the onset of adverse events and treatment feasibility. Twenty-three patients (13 males), median age 62 yr (range 32–87) were considered for the study. All patients were affected by WD metastatic NET and had tumor progression in the last 6 months before the enrolment in the study. Median duration of response was 28 months (range 6–50 months). Fourteen patients (60.9%) showed flushing and diarrhea which improved by 85.7% and 55.6%, respectively, bronchoconstrinction and abdominal pain also ameliorated. A complete, partial or no-changed response in the tumor markers behavior was observed, respectively, in 42.9%, 22.9%, and 17.1% of cases. According to RECIST (Response Evaluation Criteria In Solid Tumors) criteria (version 1.1), there were 2 partial regression (8.7%) and 15 stable disease (65.3%); 6 patients (26.0%) progressed. No patient complained from any severe adverse reaction. The results of our study suggest that lanreotide autogel is effective in the symptoms, biochemical markers, and tumor progression control of WD metastatic NET and confirm that the treatment is well tolerated.
Similar content being viewed by others
References
Oberg K, Akerström G, Rindi G, Jelic S; ESMO Guidelines Working Group. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010, 21 (Suppl): v223–7.
Oberg K, Hellman P, Kwekkeboom D, Jelic S; ESMO Guidelines Working Group. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010, 21 (Suppl): v220–2.
Boudreaux JP. Surgery for gastroenteropancreatic neuroendocrine tumors (GEPNETS). Endocrinol Metab Clin North Am 2011, 40: 163–71.
Joseph S, Wang YZ, Boudreaux JP, et al. Neuroendocrine tumors: current recommendations for diagnosis and surgical management. Endocrinol Metab Clin North Am 2011, 40: 205–31.
Steinmüller T, Kianmanesh R, Falconi M, et al.; Frascati Consensus Conference participants. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2008, 87: 47–62.
Oberg K, Ferone D, Kaltsas G, Knigge UP, Taal B, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biotherapy. Neuroendocrinology 2009, 90: 209–13.
Eriksson B, Annibale B, Bajetta E, et al; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology 2009, 90: 214–9.
Raut CP, Kulke MH. Targeted therapy in advanced well-differentiated neuroendocrine tumors. Oncologist 2011, 16: 286–95.
Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and non-functional neuroendocrine tumours. Cancer 1993, 72: 244–8.
Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut 1996, 38: 430–8.
Di Bartolomeo M, Bajetta E, Buzzoni R, et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer 1996, 77: 402–8.
Culler MD, Oberg K, Arnold R, Krenning EP, Sevilla I, Díaz JA. Somatostatin analogs for the treatment of neuroendocrine tumors. Cancer Metastasis Rev 2011, 30 (Suppl): 9–17.
Garland J, Buscombe JR, Bouvier C, et al. Sandostatin LAR (long-acting octreotide acetate) for malignant carcinoid syndrome: a 3-year experience. Aliment Pharmacol Ther 2003, 17: 437–44.
Wymenga AN, Eriksson B, Salmela PI, et al. Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumours and hormone-related symptoms. J Clin Oncol 1999, 17: 1111.
Oberg KE, Reubi JC, Kwekkeboom DJ, Krenning EP. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010, 139: 742–53.
Clements D, Elias E. Regression of metastatic vipoma with somatostatin analogue SMS 201-995. Lancet 1985, 1: 874–5.
Imtiaz KE, Monteith P, Khaleeli A. Complete histological regression of metastatic carcinoid tumour after treatment with octreotide. Clin Endocrinol (Oxf) 2000, 53: 755–8.
Shepherd JJ, Senator GB. Regression of liver metastases in patient with gastrin-secreting tumour treated with SMS 201-995. Lancet 1986, 2: 574.
Granberg D, Jacobsson H, Oberg K, Gustavsson J, Lehtihet M. Regression of a large malignant gastrinoma on treatment with Sandostatin LAR: a case report. Digestion 2008, 77: 92–5.
Rinke A, Müller HH, Schade-Brittinger C, et al. PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009, 27: 4656–63.
Castellano D, Salazar R, Raymond E. Future perspectives on neuroendocrine tumors. Cancer Metastasis Rev 2011, 30 (Suppl): 35–40.
Eriksson B, Renstrup J, Imam H, Oberg K. High-dose treatment with lanreotide of patients with advanced neuroendocrine gastrointestinal tumours: clinical and biological effects. Ann Oncol 1997, 8: 1041–4.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009, 45: 228–47.
Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of tumours of the digestive system. Lyon: IARC Press, 2010.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004.
Bajetta E, Procopio G, Catena L, et al. Lanreotide autogel every 6 weeks compared with Lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: a Phase III Study. Cancer 2006, 107: 2474–81.
Arnold R, Wilke A, Rinke A, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol 2008, 6: 820–7.
Ducreux M, Ruszniewski P, Chayvialle JA, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol 2000, 95: 3276–81.
Ruszniewski P, Ish-Shalom S, Wymenga M, et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology 2004, 80: 244–51.
Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 2001, 37: 1014–9.
Leong WL, Pasieka JL. Regression of metastatic carcinoid tumors with octreotide therapy: two case reports and a review of the literature. J Surg Oncol 2002, 79: 180–7.
Bondanelli M, Ambrosio MR, Zatelli MC, Cavazzini L, Al Jandali Rifa’y L, degli Uberti EC. Regression of liver metastases of occult carcinoid tumor with slow release lanreotide therapy. World J Gastroenterol 2005, 11: 2041–4.
Giustina A, Bonadonna S, Bugari G, et al. High-dose intramuscular octreotide in patients with acromegaly inadequately controlled on conventional somatostatin analogue therapy: a randomised controlled trial. Eur J Endocrinol 2009, 161: 331–8.
Wuster C, Both S, Cordes U, Omran W, Reisch R. Primary treatment of acromegaly with high-dose lanreotide: a case series. J Med Case Reports 2010, 4: 85.
Welin SV, Janson ET, Sundin A, et al. High-dose treatment with a long acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol 2004, 151: 107–12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Antonio Bianchi and Laura De Marinis contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bianchi, A., De Marinis, L., Fusco, A. et al. The treatment of neuroendocrine tumors with long-acting somatostatin analogs: A single center experience with lanreotide autogel. J Endocrinol Invest 34, 692–697 (2011). https://doi.org/10.3275/8058
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3275/8058