Abstract
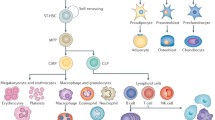
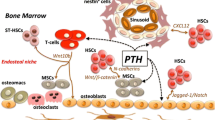
At least 2 different types of cells, hematopoietic and mesenchymal, are present in the adult bone marrow, in addition to endothelial cells. Hematopoietic and mesenchymal cells are believed to originate from hematopoietic stem cells (HSC) and mesenchymal stem cells (MSC), respectively. The bone marrow stroma, a cellular microenvironment that supports HSC, is composed of non-hematopoietic cells and contains MSC. A unique expansion of the bone marrow stroma, also known as marrow fibrosis, is the hallmark of a variety of disorders including hyperparathyroidism and fibrous dysplasia. PTH is the first bone anabolic agent approved by US Food and Drug Administration for the treatment of osteoporosis. Recent studies have suggested that PTH treatment may affect the number of hematopoietic stem cells in the bone marrow and their mobilization into the bloodstream. In addition, cells with classical features of mesenchymal stem cells/progenitors have been shown to express receptors for PTH, and to increase in number and undergo redistribution in the adult bone marrow upon PTH treatment. In this review, we will summarize the up-to-date knowledge on PTH and its relation to stem cells. We will also discuss the contribution of different cell types to the development of marrow fibrosis and the involvement of PTH signaling in this pathology.
Similar content being viewed by others
References
Wu AM, Till JE, Siminovitch L, McCulloch EA. Cytological evidence fora relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med 1968, 127: 455–64.
Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1994, 1: 661–73.
Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A 1992, 89: 1502–6.
Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121: 1109–21.
Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970, 3: 393–403.
Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17: 331–40.
Caplan AI. Mesenchymal stem cells. J Orthop Res 1991, 9: 641–50.
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284: 143–7.
Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131: 324–36.
Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466: 829–34.
Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res 2003, 34: 565–71.
Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009, 15: 757–65.
Ohishi M, Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem 2010, 109: 277–82.
Olmsted-Davis EA, Gugala Z, Camargo F, et al. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci U S A 2003, 100: 15877–82.
Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood 2006, 108: 2893–6.
Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007, 87: 858–70.
Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol 2003, 74: 833–45.
Gensure RC, Gardella TJ, Jüppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun 2005, 328: 666–78.
Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 2008, 29: 155–92.
Lotinun S, Sibonga JD, Turner RT. Evidence that the cells responsible for marrow fibrosis in a rat model for hyperparathyroidism are preosteoblasts. Endocrinology 2005, 146: 4074–81.
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4: 7–25.
Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425: 841–6.
Zhang J, Niu C, Ye L, Huang H, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 2003: 836–41.
Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004, 118: 149–61.
Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124: 407–21.
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004, 103: 3258–64.
Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol 2006, 7: 333–7.
Yin T, Li L. The stem cell niches in bone. J Clin Invest 2006, 116: 1195–201.
Calvi LM. Osteoblastic activation in the hematopoietic stem cell niche. Ann N YAcad Sci 2006, 1068: 477–88.
Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol 2007, 25: 238–43.
Ballen KK, Shpall EJ, Avigan D, et al. Phase I trial of parathyroid hormone to facilitate stem cell mobilization. Biol Blood Marrow Transplant 2007, 13: 838–43.
Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010, 33: 387–99.
Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003, 349: 1207–15.
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 2003, 349: 1216–26.
Wu X, Pang L, Lei W, et al. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 2010, 7: 571–80.
Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol 2007, 139: 351–62.
Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 1993, 328: 171–5.
Kumbasar B, Taylan I, Kazancioglu R, Agan M, Yenigun M, Sar F. Myelofibrosis secondary to hyperparathyroidism. Exp Clin Endocrinol Diabetes 2004, 112: 127–30.
Lim DJ, Oh EJ, Park CW, et al. Pancytopenia and secondary myelofibrosis could be induced by primary hyperparathyroidism. Int J Lab Hematol 2007, 29: 464–8.
Riminucci M, Robey PG, Saggio I, Bianco P. Skeletal progenitors and the GNAS gene: fibrous dysplasia of bone read through stem cells. J Mol Endocrinol 2010, 45: 355–64.
Turner RT, Iwaniec UT, Marley K, Sibonga JD. The role of mast cells in parathyroid bone disease. J Bone Miner Res 2010, 25: 1637–49.
Calvi LM, Sims NA, Hunzelman JL, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells. differentially affects cortical and trabecular bone. J Clin Invest 2001, 107: 277–86.
Halasy-Nagy JM, Rodan GA, Reszka AA. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 2001, 29: 553–9.
Ohishi M, Chiusaroli R, Ominsky M, et al. Osteoprotegerin abrogated cortical porosity and bone marrow fibrosis in a mouse model of constitutive activation of the PTH/PTHrP receptor. Am J Pathol 2009, 174: 2160–71.
Yamamoto T, Ozono K, Kasayama S, et al. Increased IL-6-production by cells isolated from the fibrous bone dysplasia tissues in patients with McCune-Albright syndrome. J Clin Invest 1996, 98: 30–5.
Piersanti S, Remoli C, Saggio I, et al. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res 2010, 25: 1103–16.
Glorieux FH, Rauch F. Medical therapy of children with fibrous dysplasia. J Bone Miner Res 2006, 21(Suppl 2): P110–3.
Chapurlat RD. Medical therapy in adults with fibrous dysplasia of bone. J Bone Miner Res 2006, 21(Suppl 2): P114–9.
Calvi LM, Schipani E. The PTH/PTHrP receptor in Jansen’s metaphyseal chondrodysplasia. J Endocrinol Invest 2000, 23: 545–54.
Guo J, Liu M, Yang D, et al. Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology 2010, 151: 3502–13.
Guo J, Liu M, Yang D, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 2010, 11: 161–71.
Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep 2008, 6: 72–6.
Dattta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal 2009, 21: 1245–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohishi, M., Schipani, E. PTH and stem cells. J Endocrinol Invest 34, 552–556 (2011). https://doi.org/10.3275/7620
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3275/7620