Abstract
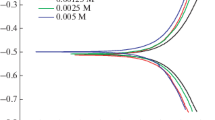
The present work describes the corrosion inhibition of mild steel—one of the metals widely utilized in industrial processes, in 0.25 M hydrochloric acid, using an amide substituted macrocyclic zinc phthalocyanine (TAZnPc). The corrosion inhibition efficiency was studied via potentiodynamic polarization technique and electrochemical impedance spectroscopy in a temperature range of 303 to 323 K, with the concentration of the inhibitor of 0.625–2.5 mM. The electrochemical study reveals that TAZnPc acts as mixed inhibitor, and the inhibition efficiency was found to increase with increasing the inhibitor concentration and decreasing temperature. The studied inhibitor showed the utmost inhibition efficiency of 86.48% at its optimum concentration of 5 mM. The excellent inhibitory performance is attributed to both the physisorption and chemisorption processes of adsorption of TAZnPc on the surface of mild steel. It was found that it followed the Langmuir adsorption isotherm. The results obtained by both potentiodynamic polarization technique and electrochemical impedance spectroscopy methods were in good agreement with each other. The surface morphology of the mild steel surface was studied by taking scanning electron microscope images, energy-dispersive X-ray spectroscopy and atomic force spectroscopy images without and with TAZnPc in 0.25 M HCl.










Similar content being viewed by others
REFERENCES
Philip, A.S., Fundamentals of Metallic Corrosion, Boca Raton, FL: CRC Press, 2006, p. 2.
Ishtiaque, A., Rajendra, P., and Quraishi, M.A., Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions, Corros. Sci., 2010, vol. 52, no. 3, p. 933. https://doi.org/10.1016/j.corsci.2009.11.016
Bentiss, F., Lebrini, M., and Lagrenée, M., Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel//2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system, Corros. Sci., 2005, vol. 47, no. 12, p. 2915. https://doi.org/10.1016/j.corsci.2005.05.034
Chauhan, L.R. and Gunasekaran, G., Corrosion inhibition of mild steel by plant extract in dilute HCl medium, Corros. Sci., 2007, vol. 49, no. 3, p. 1143. https://doi.org/10.1016/j.corsci.2006.08.012
Ashassi-Sorkhabi, H., Shaabani, B., and Seifzadeh, D., Effect of some pyrimidinic schiff bases on the corrosion of mild steel in hydrochloric acid solution, Electochim. Acta, 2005, vol. 50, nos. 16–17, p. 3446. https://doi.org/10.1016/j.electacta.2004.12.019
Sundaram, R.G. and Sundaravadivelu, M., Surface protection of mild steel in acidic chloride solution by 5‑nitro-8-hydroxy quinoline, Egypt. J. Petrol., 2018, vol. 27, no. 1, p. 95. https://doi.org/10.1016/j.ejpe.2017.01.008
Zaher, A., Chaouiki, A., Salghi, R., Boukhraz, A., et al., Inhibition of mild steel corrosion in 1 M hydrochloric medium by the methanolic extract of Ammi visnaga L. lam seeds, Int. J. Corros., 2020, vol. 2020, p. 9764206. https://doi.org/10.1155/2020/9764206
Kamali, E., Kowsari, E., and Ehsani, A., Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study, Colloids Surf. A, 2020, vol. 586, p. 124195. https://doi.org/10.1016/j.colsurfa.2019.124195
Aquino-Torres, E., Camacho-Mendoza, R.L., Gutierrez, E., Rodriguez, J.A., et al., The influence of iodide in corrosion inhibition by organic compounds on carbon steel: Theoretical and experimental studies, Appl. Surf. Sci., 2020, vol. 514, p. 145928. https://doi.org/10.1016/j.apsusc.2020.145928
Valdez-Salas, B., Schorr-Wiener, M., and Cheng, N., Vapor phase corrosion inhibitors for oil and gas field, in Corrosion Inhibitors in the Oil and Gas Industry, Viswanathan, S.S. and Saviour, A.U., Eds., Weingeim: Wiley, 2020, p. 339. https://doi.org/10.1002/9783527822140.ch14
Dinh, Q.H., Nguyen, T.L.H., Tran, T.A.N., Tran, D., et al., Pivote role of heteroatoms in improving the corrosion inhibition ability of thiourea derivatives, ACS Omega, 2020, vol. 42, p. 27655. https://doi.org/10.1021/acsomega.0c04241
Chaouiki, A., Lgaz, H., Salghi, R., Chafiq, et al., Assessing the impact of electron-donating-substituted chalcones on inhibition of mild steel corrosion in HCl solution: Experimental results and molecular-level insights, Colloids Surf. A, 2020, vol. 588, p. 124366. https://doi.org/10.1016/j.colsurfa.2019.124366
Olivares-Xometi, O., Likhanova, N.V., Domingues-Aguilar, M.A., Hallen, J.M., et al., Surface analysis of inhibitor films formed by imidazolines and amides on mild steel in an acidic environment, Appl. Surf. Sci., 2006, vol. 252, no. 6., p. 2139. https://doi.org/10.1016/j.apsusc.2005.03.178
Obot, I.B., Onyeachu, I.B., and Umoren, S.A., Pyrazines as potential corrosion inhibitors for industrial metals and alloys: A review, J. Bio- Tribo-Corros., 2018, vol. 4, no. 2, p. 18. https://doi.org/10.1007/s40735-018-0135-2
Xianghong, L., Shuduan, D., and Hui, F., Adsorption and inhibition effect of vanillin on cold-rolled steel in 3.0 M H3PO4, Progr. Org. Coat., 2010, vol. 67, no. 4, p. 420. https://doi.org/10.1016/j.porgcoat.2009.12.006
Walter, M.G., Rudine, A.B., and Wamser C., Porphyrins and phthalocyanines in solar photovoltaic cells, J. Porphyr. Phthalocyanines, 2010, vol. 14, p. 759. https://doi.org/10.1142/S1088424610002689
Rella, R., Serra, A., Siciliano, P., Tepore, A., et al., Langmuir–Blodgett multilayers based on copper phthalocyanine as gas sensor materials: Active layer–gas interaction model and conductivity modulation, Langmuir, 1997, vol. 13, no. 24, p. 6562. https://doi.org/10.1021/la961029c
Al-Sohaimi, B.R., Pişkin, M., Aljuhani, A., Al-Raqa, S.Y., et al., Enhancing photophysical and photochemical properties of zinc(II) phthalocyanine dyes by substitution of triptycene moieties, J. Lumin., 2015, vol. 173, p. 82. https://doi.org/10.1016/j.jlumin.2015.12.053
Gregory, P., Industrial applications of phthalocyanines, J. Porphyr. Phthalocyanines, 2000, vol. 4, no. 4, p. 432. https://doi.org/10.1002/(SICI)1099-1409(200006/07)4:4<432::AID-JPP254>3.0.CO;2-N
Dibestoe, M., Olasunkanmi, L.O., Fayemi, O.E., Yesudass, S.A., et al., Some phthalocyanine and naphthalocyanine derivatives as corrosion inhibitors for aluminum in acidic medium: experimental, quantum chemical calculations, QSAR studies and synergistic effect of iodide ions, Molecules, 2015, vol. 20, no. 9, p. 15701. https://doi.org/10.3390/molecules200915701
Zhao, P., Liang, Q., and Li, Y., Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/L HCl, Appl. Surf. Sci., 2005, vol. 252, no. 5, p. 1596. https://doi.org/10.1016/j.apsusc.2005.02.121
Aoki, I.V. Guedes, I.C., and Maranhao, S.L.A., Copper phthalocyanine as corrosion inhibitor for ASTM A606-4 steel in 16% hydrochloric acid, J. Appl. Electrochem., 2002, vol. 32, no. 8, p. 915. https://doi.org/10.1023/A:1020506432003
Ozdemir, O.K., Aytac, A., Atilla, D., and Durmus, M., Corrosion inhibition of aluminum by novel phthalocyanines in hydrochloric acid solution, J. Mater. Sci., 2011, vol. 46, no. 3, p. 752. https://doi.org/10.1007/s10853-010-4808-6
Song, X., She, Y., Ji, H., and Zhang, Y., Highly efficient, mild, bromide-free and acetic acid-free dioxygen oxidation of p-nitrotoluene to p-nitrobenzoic acid with metal phthaclocyanine catalysts, Org. Process. Res. Dev., 2005, vol. 9, no. 3, p. 297. https://doi.org/10.1021/op049810b
Shahin, M., Bilgic, S., and Yilmaz, H., The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl medium, Appl. Surf. Sci., 2002, vol. 195, no. 1, p. 1. https://doi.org/10.1016/S0169-4332(01)00783-8
Abd el Kader, J.M., El Warraky, A.A., and Abd el Aziz, A.M., Corrosion inhibition of mild steel by sodium tungstate in neutral solution. Part 1: Behaviour in distilled water, Br. Corros. J., 1998, vol. 33, no. 2, p. 139. https://doi.org/10.1179/000705998798115524
Li, W., He, Q., Pei C., and Hou B.R., Experimental and theoretical investigation of the adsorption behavior of new triazole derivatives as inhibitors for mild steel corrosion in acid media, Electrochim. Acta, 2007, vol. 52, no. 22, p. 6386. https://doi.org/10.1016/j.electacta.2007.04.077
Wei-Hua, L., Qiao, H., Sheng-Tao, Z., Chang-Ling, P., et al., Some new triazole derivatives as inhibitors for mild steel corrosion in acid medium, J. Appl. Electrochem., 2008, vol. 38, no. 3, p. 289. https://doi.org/10.1007/s10800-007-9437-7
Machnikova, E., Whitmire, K.H., and Hackerman, N., Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives, Electrochim. Acta, 2008, vol. 53, no. 20, p. 6024. https://doi.org/10.1016/j.electacta.2008.03.021
Singh, A.K. and Quraishi, M.A., Effect of cefazolin on the corrosion of mild steel in HCl solution, Corros. Sci., 2010, vol. 52, no. 1, p. 152. https://doi.org/10.1016/j.corsci.2009.08.050
Ashassi-Sorkhabi, H., Majidi, M.R., and Seyyedi, K., Investigation of inhibition effect of some amino acids against steel corrosion in HCl solution, Appl. Surf. Sci., 2004, vol. 225, nos. 1–4, p. 176. https://doi.org/10.1016/j.apsusc.2003.10.007
Bouklah, M., Hammouti, B., Lagrenee, M., and Bentiss, F., Thermodynamic properties of 2,5-bis(4-methoxyphenyl)-1,3,4-oxadiazole as corrosion inhibitor for mild steel in normal sulfuric acid medium, Corros. Sci., 2006, vol. 48, no. 9, p. 2831. https://doi.org/10.1016/j.corsci.2005.08.019
Durnie, W.H., Kinsella, B.J., de Marco, R., and Jefferson, A., A study of adsorption properties of commercial carbon dioxide corrosion inhibitor formulations, J. Appl. Electrochem., 2001, vol. 31, no. 11, p. 1221. https://doi.org/10.1023/A:1012716911305
Kobayashi, T., Kurokawa, F., Uyeda, N., and Suito, E., The metal ligand vibration in the infrared spectra of various metal phthalocyanines, Spectrochim. Acta A Mol. Biomol. Spectrosc., 1970, vol. 26, no. 6, p. 1305. https://doi.org/10.1016/0584-8539(70)80036-8
Bayo, K., Mossoyan, J.C., and Ouedraogo, G.V., Preparation and analysis by UV-Vis of zinc phthalocyanine complexes, Spectrochim. Acta A, 2004, vol. 60, no. 3, p. 653. https://doi.org/10.1016/S1386-1425(03)00275-0
Khaled, K.F. and Hackerman, N., Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions, Electrochem. Acta, 2003, vol. 48, no. 19, p. 2715. https://doi.org/10.1016/S0013-4686(03)00318-9
ACKNOWLEDGMENTS
Sarvajith Malali Sudhakara acknowledges the Manipal Academy of Higher Education, India, for providing the TMA Pai fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Khan, F., Sudhakara, S.M., Puttaigowda, Y.M. et al. Amide Substituted Zinc Centered Macrocyclic Phthalocyanines for Corrosion Inhibition of Mild Steel in Hydrochloric Acid Medium. Surf. Engin. Appl.Electrochem. 58, 613–624 (2022). https://doi.org/10.3103/S1068375523010076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375523010076