Abstract
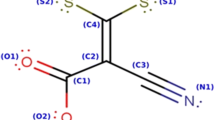
A comparative study of Fe and Cr complexes with a Schiff-base ligand (1-[(2-hydroxyethyl)amino]-2-(salicylideneamino)ethane) as inhibitors for carbon steel corrosion in 0.5 M HCl solution at 25°C was carried out. Corrosion measurements based on the weight loss method, potentiodynamic polarization curves, and electrochemical impedance spectroscopy (EIS) indicate that Fe complex, in most cases, accelerates carbon steel corrosion in HCl while Cr complex act as moderately inhibitor. The UV-visible and the attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR) studies of the electrode surface confirmed existence of an adsorbed film of the complexes on the electrode surface. Adsorption of Cr complex obeys the Langmuir adsorption isotherm with a very low value of free energy of adsorption ΔG° (physisorption). Cr complex acts as mixed type inhibitor, with the dominanting effect on the anodic dissolution of iron. The mechanism of adsorption is discussed and it is concluded that the adsorbed chloride ions on the metal surface could be stabilized in theiranionic form by hydrogen bonding of oxygen and nitrogen atoms in the tail of the complexes, like a crab-claw. The accelerating properties of Fe complex could be due to the formation of non adherent and/or soluble complex that is readily removed from the metal surface.
Similar content being viewed by others
References
Sari, N. and Gürkan, P., Synthesis, potentiometric and antimicrobial activity studies on 2-pyridinilidene-DL-amino acids and their complexes, Trans. Met. Chem., 2003, vol. 28, pp. 468–474.
Ehteshamzadeh, M., Shahrabi, T., and Hosseini, M., Innovation in acid pickling treatments of copper by characterizations of a new series of Schiff bases as corrosion inhibitors, Anti-Corr. Method, Ser. M, 2006, vol. 53, pp. 296–302.
Kustu, C., Emregul, K.C., and Atakol, O., Schiff bases of increasing complexity as mild steel corrosion inhibitors in 2 M HCl, Corr. Sci., 2007, vol. 49, pp. 2800–2814.
Youssef, S.N., El-Zahany, E., El-Seidy, A.M.A., Caselli, A., and Cenin, S., Synthesis and characterization of some transition metal complexes with a novel Schiff base ligand and their use as catalysts for olefin cyclopropanation, J. Mol. Catal., Ser. A, 2009, vol. 308, pp. 159–165.
Patil, A.S., Naika, H.V., Kulkarnia, D.A., and Badami, S.P., DNA cleavage, antimicrobial, spectroscopic and fluorescence studies of Co(II), Ni(II) and Cu(II) complexes with SNO donor coumarin Schiff bases, Spectrochim. Acta, Ser. A, 2010, vol. 75, pp. 347–354.
Devi, I.G., Parameswaran, G., and Veena, V., Synthesis and characterization of lanthanide(III) perchlorate complexes of some Schiff base ligands, Asian J. Chem., 2004, vol. 16, pp. 493–500.
Sheng, X., Guo, X., Lu, M.X., Lu, Y.G., Shao, Y., Liu, F., and Xu, Q., DNA binding, cleavage, and cytotoxic activity of the preorganized dinuclear zinc(II) complex of triazacyclononane derivatives, Bioconjugate Chem., 2008, vol. 19, pp. 490–498.
Valentovic, M.A. and Ball, J.G., 2-Aminophenol and 4-aminophenol toxicity in renal slices from Spraguedaeley and Fischer 344 rats, J. Toxicol. Env. Heal., Ser. A, 1998, vol. 55, pp. 225–240.
Song, H. and Chen, T.S., p-Aminophenol-induced liver toxicity: Tentative evidence of a role for acetaminophen, J. Biochem. Mol. Toxicol., 2001, vol. 15, no. 1, pp. 34–40.
Issaadi, S., Douadi, T., Zouaoui, A., Chafaa, S., Khan, M.A., and Boue, G., Novel thiophene symmetrical Schiff base compounds as corrosion inhibitor for mild steel in acidic media, Corr. Sci., 2011, vol. 53, pp. 1484–1488.
Nathan, C.C., Organic Inhibitors, NACE, Houston, TX, 1977, p. 7.
Behpour, M., Ghoreishi, S.M., Soltani, N., and Salavati-Niasari, M., The inhibitive effect of some bis-N,Sbidentate Schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution, Corr. Sci., 2009, vol. 51, pp. 1073–1082.
Naderi, E., Jafari, A.H., Ehteshamzadeh, M., and Hoseini, M.G., Effect of carbon steel microstructures and molecular structure of two new Schiff base compounds on inhibition performance in 1 M HCl solution by EIS, Mater. Chem. Phys., 2009, vol. 115, pp. 852–858.
Behpor, M., Ghoreishi, S.M., Soltani, N., Salavati-Niasari, M., Hamadanian, M., and Gandomi, A., Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution, Corr. Sci., 2008, vol. 50, pp. 2172–2181.
Aramaki, K., Node, Y., and Nishihara, H., Adsorption and corrosion inhibition effect of polar organic compounds on iron in 1 M HClO4 containing SH, J. Electrochem. Soc., 1990, vol. 137, pp. 1354–1358.
Rangelov, S. and Mircheva, V., The influence of metal complexes of tetramethyldithio-oxamide on the rate of acid corrosion of steel, Corr. Sci., 1996, vol. 38, pp. 301–306.
Khaled, K.F., Babic-Samardzija, K., and Hackerman, N., Cobalt(III) complexes of macrocyclic-bidentate type as a new group of corrosion inhibitors for iron in perchloric acid, Corr. Sci., 2006, vol. 48, pp. 3014–3034.
Abdel-Gaber, A.M., Masoud, M.S., Khalil, E.A., and Shehata, E.E., Electrochemical study on the effect of Schiff base and its cobalt complex on the acid corrosion of steel, Corr. Sci., 2009, vol. 51, pp. 3021–3024.
Sari, N. and Aytac, A., Mechanistic Ssudy of Ir(III) catalyzed oxidation of amines by acidic solution of potassium bromate, Asian J. Chem., 2009, vol. 21, pp. 839–848.
Ouf, A.E.-F.M., Ali, M.S., Soliman, M.S., El-Defrawy, A.M., and Mostafa, S.I., Synthesis and characterization of new transition metal complexes of Schiff-base derived from 2-aminopyrimidine and 2,4dihydroxybenzaldehyde and its applications in corrosion inhibition, J. Korean. Chem. Soc., 2010, vol. 54, pp. 402–418.
Nasr-Esfahani, M., Zendehdel, M., and Jafari, B., Electro-chemical and X-ray structural study of corrosion inhibition and adsorption behavior for mild steel by a new Schiff base cobalt complex in HCl, Prot. Met. Phys. Chem. Surf., 2015, vol. 51, pp. 285–294.
Pourriahi, M., Nasr-Esfahani, M., and Motalebi, A., Effect of henna and rosemary extracts on the corrosion of 304L stainless steel in 3.5% NaCl solution, Surf. Eng. Appl. Electrochem., 2014, vol. 50, pp. 525–533.
Mikuriya, M. and Matsunami, K., Synthesis and structural characterization of a series of transition metal complexes with a tetradentate Schiff-base ligand derived from salicylaldehyde and 2-(2-aminoethylamino)ethanol, Mater. Sci.-Poland, 2005, vol. 23, no. 3, pp. 773–792.
ASTM, Standard Practice for Laboratory Immersion Corrosion Testing of Metals, 3.02, G 31–72, Annual Book of Standards, 1990.
Obot, I.B., Obi-Egbedi, N.O., and Umoren, S.A., Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl, Corr. Sci., 2009, vol. 51, pp. 1868–1875.
Migahed, M.A. and Nassar, I.F., Corrosion inhibition of tubing steel during acidization of oil and gas wells, Electrochim. Acta, 2008, vol. 53, pp. 2877–2882.
Bentiss, F., Gassama, F., Barbary, D., Gengembre, L., Vezin, H., Laggrenee, M., and Traisnel, M., Appl. Surf. Sci., 2006, vol. 252, pp. 2684–2691.
Quraishi, M.A. and Rawat, J., Corrosion inhibiting action of tetramethyl-dithia-octaaza-cyclotetradecahexaene (MTAH) on corrosion of mild steel in hot 20% sulfuric acid, Mater. Chem. Phys., 2003, vol. 77, pp. 43–47.
Lopez, D.A., Simison, S.N., and de Sanchez, S.R., The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole, Electrochim. Acta, 2003, vol. 48, pp. 845–854.
Abdel-Gabar, A.M., Abd-El-Nabey, B.A., Sidahmed, I.M., El-Zayady, A.M., and Saadawy, M., Inhibitive action of some plant extracts on the corrosion of steel in acidic media, Corr. Sci., 2006, vol. 48, pp. 2765–2779.
Macdonald, J.R. and Johanson, W.B., Theory in Impedance Spectroscopy, NY: John Wiley & Sons, 1987, p. 45.
Mansfeld, F., Kendig, M.W., and Tsai, S., Recording and analysis of AC impedance data for corrosion studies, Corrosion, 1982, vol. 38, p. 570.
Lenderrink, H.J.W., Linden, M.V.D., and De Wit, J.H.W., Corrosion of aluminium in acidic and neutral solutions, Electrochim. Acta, 1993, vol. 38, pp. 1989–1992.
Sherif, E.M. and Park, S.-M., Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions, Electrochim. Acta, 2006, vol. 51, pp. 1313–1321.
Abd El-Maksoud, S.A. and Hassan, H.H., Electrochemical studies on the effect of (2E)-3-amino-2-phenylazo-but-2-enenitrile and its derivative on the behaviour of copper in nitric acid, Mater. Corr., 2007, vol. 58, pp. 369–375.
Hsu, C.H. and Mansfeld, F., Technical note: concerning the conversion of the constant phase element parameter Y0 into a capacitance, Corrosion, 2001, vol. 57, p. 747.
Migahed, M.A., Electrochemical investigation of the corrosion behaviour of mild steel in 2 M HCl solution in presence of 1-dodecyl-4-methoxy pyridinium bromide, Mater. Chem. Phys., 2005, vol. 93, pp. 48–53.
Hassan, H., Abdelghani, E., and Amin, M., Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives, Part I: Polarization and EIS studies, Electrochim. Acta, 2007, vol. 52, pp. 6359–6366.
Hassan, H.H., Perchlorate and oxygen reduction during Zn corrosion in a neutral medium, Electrochim. Acta, 2006, vol. 51, p. 5966.
Noor, E.A. and Al-Moubaraki, A.H., Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4'(-X)-styryl pyridinium iodides/hydrochloric acid systems, Mater. Chem. Phys., 2008, vol. 110, pp. 145–154.
Avci, G., Corrosion inhibition of indole-3-acetic acid on mild steel in 0.5 M HCl, Colloid Surf., Ser. A, 2008, vol. 317, nos. 1–3, pp. 730–736.
Langmuir, I., The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc., 1916, vol. 38, pp. 2221–2295.
Morad, M.S., Inhibition of phosphoric acid corrosion of zinc by organic onium compounds and their adsorption characteristics, J. Appl. Electrochem., 1999, vol. 29, pp. 619–626.
Morad, M.S. and Sarhan, A.A., Application of some ferrocene derivatives in the field of corrosion inhibition, Corr. Sci., 2008, vol. 50, pp. 744–753.
Avci, G., Corrosion inhibition of indole-3-acetic acid on mild steel in 0.5 M HCl, Colloid Surf., Ser. A, 2008, vol. 317, pp. 730–736.
Pandey, A., Murty, N.S.S., and Patel, S.M., Application of infrared spectroscopy in the study of corrosion products, Process. Contr. Qual., 1999, vol. 11, p. 363.
Popova, A., Sokolova, E., Raicheva, S., and Christova, M., AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives, Corr. Sci., 2003, vol. 45, pp. 33–58.
Benerijee, G. and Malhotra, S.N., Contribution to adsorption of aromatic amines on mild steel surface from HCl solutions by impedance, UV, and Raman spectroscopy, Corrosion, 1992, vol. 48, pp. 10–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Kord, L., Nasr-Esfahani, M. Corrosion behavior of carbon steel in HCl solution by Fe and Cr complexes with a Schiff-base ligand derived from salicylaldehyde and 2-(2-aminoethylamino)ethanol. Surf. Engin. Appl.Electrochem. 51, 491–500 (2015). https://doi.org/10.3103/S1068375515050087
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375515050087