Abstract
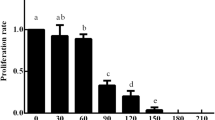
Experiments were carried out to compare the efficiency of embryogenic carrot calli transformation using selective genes nptII and hpt. Our data showed that it is more preferable to use kanamycin concentration 100 mg/L and hygromycin 20 mg/L for the selection of transgenic shoots. Under the same conditions, the use of kanamycin yielded a greater number of transgenic shoots. It is, therefore, more preferable to use vector constructs containing gene nptII for genetic transformation of the Nantskaya 4 carrot cultivar.
Similar content being viewed by others
References
Scott, R.J. and Draper, J., Transformation of carrot tissues derived from proembryogenic suspension cells: A useful model system for gene expression studies in plants, Plant Mol. Biol., 1987, vol. 8, pp. 265–274.
Baranski, R., Genetic transformation of carrot (Daucus carota L.) and other apiaceae species, Transgenic Plant J., 2008, vol. 2, pp. 18–38.
Chen, W.P. and Punja, Z.K., Transgenic herbicideand disease-tolerant carrot (Daucus carota L.) plants obtained through Agrobacterium-mediated transformation, Plant Cell Rep., 2002, vol. 20, pp. 929–935.
Wally, O., Jayaraj, J., Zamir, K., and Punja, Z.K., Comparative resistance to foliar fungal pathogens in transgenic carrot plants expreßsing genes encoding for chitinase, β-1,3-glucanase and peroxidase, Eur. J. Plant. Pathol., 2009, vol. 123, pp. 331–342.
Deineko, E.V., Zagorskaya, A.A., Pozdnyakov, S.G., Filippenko, E.A., Permyakova, N.V., Sidorchuk, Yu.V., Uvarova, E.A., Pozdnyakova, L.D., Shumnyi, V.K., Vlasov, V.V., Khemmond, R.V., and Shchelkunov, S.N., Comparative analysis of HBV M-antigen production in leaves of individual transgenic carrot plants, Dokl. Biochem. Biophys., 2009, vol. 425, no. (1), pp. 76–79.
Tyukavin, G.B., Biotechnological grounds for the selection technology of carrots: Daucus carota L., Extended Abstract of Cand. Sci. (Biol.) Dissertation, 2007.
Rogers, S. and Bendich, A., Extraction of total cellular DNA from plants, algae and fungi, in Plant Molecular Biology Manual, Dordrecht, Netherlands: Kluwer Academic Publishers, 1995.
Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W., GUS fusions: ß-glucuronidase as a sensitive and versatile gene fusion marker in higher plants, EMBO J., 1987, vol. 6, pp. 3901–3907.
Stam, M., Mol, J.N.M., and Kooter, J.M., The silence of genes in transgenic plants, Ann. Bot., 1997, vol. 79, pp. 3–12.
Serres, R., McCown, B., and Zeldin, E., Detectable ß-glucuronidase activity in transgenic cranberry is affected by endogenous inhibitors and plant development, Plant Cell Rep., 1997, vol. 16, pp. 641–646.
Vengadesan, G., Prem Anand, R., Selvaraj, N., Perl Treves, R., and Ganapathi, A., Transfer and expression of nptII and bar genes in cucumber (Cucumis satavus L.), In Vitro Cell. Dev. Biol.: Plant, 2005, vol. 41, pp. 17–21.
Miroshnichenko, D.N. and Dolgov, S.V., Influence of explant type on the efficiency of Agrobacterium-mediated transformation of remontant carnation (Dianthus caryophyllus L.) in selection on giromitsine B, Genetika, 1999, vol. 35, no. (9), pp. 1208–1213.
Weide, R., Koornneef, M., and Zabel, P.A., Simple nondestructive spraying assay for the detection of an active kanamycin resistance gene in transgenic tomato plants, Theor. Appl. Genet., 1989, vol. 78, pp. 169–172.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.N. Sidorova, D.N. Miroshnichenko, S.V. Dolgov, 2015, published in Doklady Rossiiskoi Akademii Sel’skokhozyaistvennykh Nauk, 2015, No. 3, pp. 18–22.
Presented by Academician P. N. Kharchenko
About this article
Cite this article
Sidorova, T.N., Miroshnichenko, D.N. & Dolgov, S.V. Agrobacterium-mediated transformation efficiency of carrot embryogenic callus using nptII and hpt selective genes. Russ. Agricult. Sci. 41, 216–221 (2015). https://doi.org/10.3103/S1068367415040175
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367415040175