Abstract
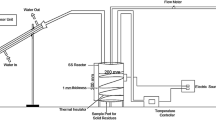
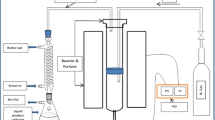
To solve the energy-saving and emission-reduction problem which caused by the deterioration of the thermal properties of coke in blast furnace and to improve the technologies applied to the inhibition to coke deterioration, benzene pyrolysis carbons were used to infiltrate into coke to inhibit coke deterioration for improving the thermal properties of coke. Taking aim at the coke reaction index (CRI) and the coke strength after reaction (CSR), the optimum infiltration conditions of benzene pyrolysis were researched by chemical vapor infiltration using response surface methodology. The models of regression of the thermal properties of coke with the experimental factors were established. Results indicated that the data were adequately fitted into quadratic models. The pyrolysis reaction time was found to have very significant linear effect on CRI and CSR. The pyrolysis reaction temperature, pyrolysis reaction time, flow rate of carrier gas and benzene temperature were found to have very significant quadratic effect on CRI and CSR, respectively. The interactions between some factors had significant effects on CRI and CSR. The experimental conditions range and optimum conditions were derived from three-dimensional response surface, contour plots and the model equations. Optimum results showed that the value of CRI decreased by about 11.94 wt % and the value of CSR increased by about 9.20 wt % under the optimum conditions. The SEM photos of cokes revealed that the pores of infiltrated coke were filled with pyrolysis carbon particles, which greatly reduced the reaction contact area for CO2 to erode coke. The changes of pore structure illustrated that the pores volume and the specific surface area of the infiltrated coke smaller than that of original coke. So infiltration of benzene pyrolysis can notably improve the thermal properties of coke.
Similar content being viewed by others
References
Yang, T.J, Zhang, J.L., and Zuo, H.B., Energy-saving, Emission-reducing and Low Carbon Ironmaking, Realizing Scientific Development of BF Production in China, China Metallurgy, 2010, no. 7, pp. 1–7.
Zhang, Z.Z., Wang, L.C., and Wang, F.A., Review of Inhibition Technology to Coke Deterioration, Chemical World, 2010, no. 7, pp. 438–442.
Bulaevskii, B.K. and Shved, V.S., Modifying CRI and CSR Values of Coke, Coke and Chemistry, 2010, no. 53, pp. 15–18.
Ulanovskii, M.L. and Miroshnichenko, D.V., Increasing the Post Reactive Strength of Coke (CSR) at the Dofasco Plant, Koks Khim., 2005, no.12, pp. 46–50.
Gornostayev, S.S. and Harkki, J.J., Mechanism of Physical Transformations of Mineral Matter in the Blast Furnace Coke with reference to Its Reactivity and Strength, Energy & Fuels, 2006, no. 20, pp. 2632–2635.
Kawano, Y., Fukuda, T., Kawarada, T., et al., Suppression of Puffing during the Graphitization of Pitch Needle Coke by Boric Acid, Carbon, 1999, no. 37, pp. 555–560.
Zhu, Z.Z., Zhang, Z.M., Tang, Q.Y., et al., Industrial Experiment on Coke Spraying with ZBS Additive in Blast Furnace, Iron & Steel Research (International), 2006, no. 13, pp.14–17.
Zhang, W.G. and Huttinger, K.J., Simulation Studies on Chemical Vapor Infiltration of Carbon, Composites Science and Technology, 2002, no. 62, pp. 1947–1955.
Shigeno, Y., Evans, J.W., and Itsumei, Y., Infiltration of Metallurgical Coke by Pyrolysis of CH4 and Its Effect on Enhancement of CSR, ISIJ International, 1998, no. 38, pp. 28–35.
Shigeno, Y. and Evans, J.W., Infiltration of Carbon in Pores within Coke and Charcoal by Methane Cracking, Metallurgical and Materials Transactions B, 1992, no. 23, pp. 429–435.
Wang, F. A. and Ren, B. Z., The Introduction to Green Process Engineering, Beijing: Chemical Industry Press, 2002.
Benito, A. M., Maniette, Y., Munoz, E., et al., Carbon Nanotubes Production by Catalytic Pyrolysis of Benzene, Carbon, 1998, no. 36, pp. 681–683.
Shih, S.I., Lin, T.C., and Shih, M.L., Decomposition of Benzene in the RF Plasma Environment Part I: Formation of Gaseous Products and Carbon Depositions, Hazardous Materials., 2004, no. B116, pp. 239–248.
Kawabuchi, Y., Kawano, S., and Mochida, I., Molecular Sieving Selectivity of Active Carbons and Active Carbon Fibers Improved by Chemical Vapour Deposition of Benzene, Carbon, 1996, no. 34, pp. 711–717.
David, E., Talaieb, A., Stanciu, V., et al., Synthesis of Carbon Molecular Sieves by Benzene Pyrolysis over Microporous Carbon Materials, Materials Processing Technology, 2004, no. 157, pp. 290–296.
Li, Y.L., Kinloch, L.A., and Windle, A.H., Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis, Science, 2004, no. 304, pp. 276–278.
Karacan, F., Ozden, U., and Karacan, S., Optimization of Manufacturing Conditions for Activated Carbon from Turkish lignite by Chemical Activation using Response Surface Methodology, Applied Thermal Engineering, 2007, no. 27, pp. 1212–1218.
Aslan, N., Application of Response Surface Methodology and Central Composite Rotatable Design for Modeling and Optimization of a Multi-gravity Separator for Chromite Concentration, Powder Technology, 2008, no. 185, pp. 80–86.
Silva, G.F., Camargo, F.L., and Ferreira, A.L.O., Application of Response Surface Methodology for Optimization of Biodiesel Production by Transesterification of Soybean Oil with Ethanol, Fuel Processing Technology, 2011, no. 92, pp. 407–413.
Box, G.E.P., Statistics for experiments: an introduction to design, data analysis and model building, New York: Wiley, 1990.
Design-Export Version 7.0 (2005), Stat-Ease, Inc. Minneapolis, MN, 55413-2726.
Pis, J.J., Menendez, J.A., Parra, J.B., et al., Relation between Texture and Reactivity in Metallurgical Cokes Obtained from Coal using Petroleum Coke as Additive, Fuel Processing Technology, 2002, no. 77, pp. 199–205.
Pusz, S., Krzesin’ska, M., Smędowski, et al., Changes in A Coke Structure Due to Reaction with Carbon Dioxide, Int. J. Coal Geology., 2010, no. 81, pp. 287–292.
Wang, L.C., Zheng, S.P., Wang, J.S., et al., Effect of Deterioration Inhibitors on Improving Coke Thermal Property, Iron and Steel, 2009, no. 5, pp. 16–18.
Xu, S.Y., Primary Calculation Research on Carbon Dioxide Emissions Benchmark of Chongqing, Chongqing: Southwest University, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Zhang, Z., Jiang, Z., Wang, L. et al. Inhibition to coke deterioration by benzene pyrolysis using response surface methodology. Coke Chem. 55, 222–230 (2012). https://doi.org/10.3103/S1068364X12060105
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X12060105