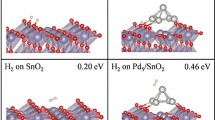
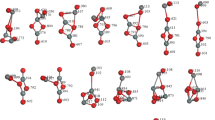
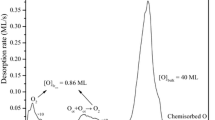
Abstract
The interaction of H2O2 with the stoichiometric surface of (110) SnO2 was studied by first principle methods. Relaxed geometries, adsorption energies, and charge transfer between the molecule and the surface were calculated for several starting configurations of the H2O2 molecule and the SnO2 surface. The most probable adsorption sites and their optimized geometries are presented.




Similar content being viewed by others
REFERENCES
Satterfield, C.N., Kavanagh, G.M., and Resnick, H., Ind. Eng. Chem., 1951, vol. 43, p. 2507.
Watt, B.E., Proudfoot, A.T., and Vale, J.A., Toxicol. Rev., 2004, vol. 23, p. 51.
Stolarek, R., Bialasiewicz, P., Krol, M., and Nowak, D., Clin. Chim. Acta, 2010, vol. 411, p. 1849.
Aroutiounian, V., Arakelyan, V., Aleksanyan, M., Gohar, S., Kacer, P., Picha, P., Kovarik, J., Pekarek, J., and Joost, B., J. Sens. Sens. Syst., 2018, vol. 7, p. 281.
Adamyan, Z.N., Sayunts, A.G., Khachaturyan, E.A., Araqelyan, V.M., Aroutiounian, V.M., and Joost, B., J. Contemp. Phys. (Armenian Ac. Sci.), 2019, vol. 54, p. 57.
Batzill, M., Sensors, 2006, vol. 6, p. 1345.
Gopel, W., Prog. Surf. Sci., 1985, vol. 20, p. 9.
Watson, J., Sensors and Actuators, 1984, vol. 5, p. 29.
Batzill, M. and Diebold, U., Phys. Chem. Chem. Phys., 2007, vol. 9, p. 2307.
Jarzebski, Z.M., J. Electrochem. Soc., 1976, vol. 123, p. 299C.
Lousada, C.M., Johansson, A.J., Brinck, T., and Jonsson, M., J. Phys. Chem. C, 2012, vol. 116, p. 9533.
Soltani, A., Peyghan, A.A., and Bagheri, Z., Phys. E Low-Dimensional Syst. Nanostructures, 2013, vol. 48, p. 176.
Plauck, A., Stangland, E.E., Dumesic, J.A., and Mavrikakis, M., Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, p. E1973.
Flätgen, G., Wasle, S., Lübke, M., Eickes, C., Radhakrishnan, G., Doblhofer, K., and Ertl, G., Electrochim. Acta, 1999, vol. 44, p. 4499.
Batzill, M., Katsiev, K., and Diebold, U., Surf. Sci., 2003, vol. 529, p. 295.
Zakaryan, H. and Aroutiounian, V., J. Contemp. Phys. (Armenian Ac. Sci.), 2017, vol. 52, p. 227.
Hunanyan, A.A., Aghamalyan, M.A., Aroutiounian, V.M., and Zakaryan, H.A., J. Contemp. Phys. (Armenian Ac. Sci.), 2019, vol. 54, p. 282.
Hohenberg, P. and Kohn, W., Phys. Rev., 1964, vol. 136, p. B864.
Kohn, W. and Sham, L.J., Phys. Rev., 1965, vol. 140, p. A1133.
Giannozzi, P., Baroni, S., Bonini, N., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Chiarotti, G.L., Cococcioni, M., Dabo, I., Dal Corso, A., De Gironcoli, S., Fabris, S., Fratesi, G., Gebauer, R., Gerstmann, U., Gougoussis, C., Kokalj, A., Lazzeri, M., Martin-Samos, L., Marzari, N., Mauri, F., Mazzarello, R., Paolini, S., Pasquarello, A., Paulatto, L., Sbraccia, C., Scandolo, S., Sclauzero, G., Seitsonen, A.P., Smogunov, A., Umari, P., and Wentzcovitch, R.M., J. Phys. Condens. Matter, 2009, vol. 21, no. 39, p. 395502.
Giannozzi, P., Andreussi, O., Brumme, T., Bunau, O., Buongiorno Nardelli, M., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Cococcioni, M., Colonna, N., Carnimeo, I., Dal Corso, A., de Gironcoli, S., Delugas, P., DiStasio, R. A., Ferretti, A., Floris, A., Fratesi, G., Fugallo, G., Gebauer, R., Gerstmann, U., Giustino, F., Gorni, T., Jia, J., Kawamura, M., Ko, H.-Y., Kokalj, A., Küçükbenli, E., Lazzeri, M., Marsili, M., Marzari, N., Mauri, F., Nguyen, N. L., Nguyen, H.-V., Otero-de-la-Roza, A., Paulatto, L., Poncé, S., Rocca, D., Sabatini, R., Santra, B., Schlipf, M., Seitsonen, A. P., Smogunov, A., Timrov, I., Thonhauser, T., Umari, P., Vast, N., Wu, X., and Baroni, S., J. Phys. Condens. Matter, 2017, vol. 29, p. 465901.
Perdew, J.P., Burke, K., and Ernzerhof, M., Phys. Rev. Lett., 1996, vol. 77, p. 3865.
Joubert, D., Phys. Rev. B - Condens. Matter Mater. Phys., 1999, vol. 59, p. 1758.
Monkhorst, H.J., and Pack, J.D., Phys. Rev. B, 1976, vol. 13, p. 5188.
Bader, R.F.W., Atoms in Molecules: A Quantum Theory,Oxford University Press, 1990.
Henkelman, G., Arnaldsson, A., and Jónsson, H., Comput. Mater. Sci., 2006, vol. 36, p. 354.
Batzill, M. and Diebold, U., Prog. Surf. Sci., 2005, vol. 79, p. 47.
Diebold, U., Surf. Sci. Rep., 2003, vol. 48, p. 53.
Schierbaum, K.D., Kirner, U.K., Geiger, J.F., and Göpel, W., Sensors Actuators B. Chem., 1991, vol. 4, p. 87.
Schierbaum, K.D., Wei-Xing, X., and Göpel, W., Berichte der Bunsengesellschaft für Phys.Chemie, 1993, vol. 97, p. 363.
Horrillo, M.C., Gutiérrez, J., Arés, L., Robla, J.I., Sayago, I., Getino, J., and Agapito, J.A., Sensors Actuators B. Chem., 1995, vol. 25, p. 507.
Yamazoe, N., Kurokawa, Y., and Seiyama, T., Sensors and Actuators, 1983, vol. 4, p. 283.
ACKNOWLEDGMENTS
The authors are also grateful to the Institute of Informatics and Automation Problems of the NAS of RA (http://cloud.asnet.am/), the EaPEC2019 project and the ‘Jülich supercomputing center’ for the computer resources provided for the calculations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
FUNDING
This work was carried out within the framework of the 19YR-2K002 (Young Scientists 2019-2021) program of thematic financing of the Scientific Committee of the Ministry of Education, Science, Culture and Sports of the Republic of Armenia.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Additional information
Translated by V. Aroutiounian
About this article
Cite this article
Aghamalyan, M.A., Hunanyan, A.A., Aroutiounian, V.M. et al. First-Principles Study of the Interaction of H2O2 with the SnO2 (110) Surface. J. Contemp. Phys. 55, 235–239 (2020). https://doi.org/10.3103/S1068337220030020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068337220030020