Abstract
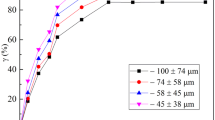
Laterite-nickel ore was roasted with ammonium sulfate to extract magnesium by a single-factor experiment, and an orthogonal test was employed to optimize the conditions. The optimum roasting conditions were: calcination temperature, 450°C; calcination time, 120 min; molar ratio of reactants, 2 : 1; granularity <80 µm. The kinetics of the roasting process were also studied. The experimental results showed that the extraction rate of magnesium increased as the calcination temperature increased. The reaction rate of magnesium is in accordance with the shrinking non-reacted nuclear model for the solid product formation reaction. The roasting reaction is controlled by internal diffusion. The apparent activation energy is E = 18.96 kJ mol–1. The kinetic equation is \({\text{1}} + {\text{2}}\left( {{\text{1}} - {\alpha\text{}}} \right) - {\text{3}}{{\left( {{\text{1}} - {\alpha\text{}}} \right)}^{{{{\text{2}} \mathord{\left/ {\vphantom {{\text{2}} {\text{3}}}} \right. \kern-0em} {\text{3}}}}}} = {\text{0}}{\text{.05061exp}}\left[ { - {{{\text{18}}{\text{963}}} \mathord{\left/ {\vphantom {{{\text{18}}{\text{963}}} {\left( {RT} \right)}}} \right. \kern-0em} {\left( {RT} \right)}}} \right]t.\) A single factor experiment and orthogonal test were carried out to optimize the magnesium leaching process reaction conditions. The optimum conditions of the leaching process were: leaching temperature, 60°C; leaching time, 60 min; liquid to solid ratio, 2.5 : 1; stirring intensity, 400 r min–1. The kinetic equation is 1 – (1 – α)2/3 = 0.3991exp(–8632/RT)t. The apparent activation energy is E = 8.63 kJ mol–1. The reaction rate of the leaching process was controlled by external diffusion.












Similar content being viewed by others
REFERENCES
Matyukhin, V.I., Shatsillo, V.V., Kuznetsov, A.V., Rybakin, D.V., and Krokhalev, A.F., Evaluating the thermal efficiency of a shaft furnace for roasting siderite ore, Metallurgist, 2017, vol. 61, pp. 3–11.
Agatzini-Leonardou, S., Tsakiridis, P.E., Oustadakis, P., Karidakis, T., and Katsiapi, A., Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores, Miner. Eng., 2009, vol. 22, pp. 1181–1192.
Valix, M., Tang, J.Y., and Cheung, W.H., The effects of mineralogy on the biological leaching of nickel laterite ores, Miner. Eng., 2001, vol. 14, pp. 1629–1635.
Zare Tavakoli, H., Abdollahy, M., Ahmadi, S. J., and Khodadadi Darban, A., The effect of particle size, irrigation rate and aeration rate on column bioleaching of uranium ore, Russ. J. Non-Ferrous Met., 2017, vol. 58, no. 3, pp. 188–199.
Coto, O., Galizia, F., Hernández, I., Marrero, J., and Donati, E., Cobalt and nickel recoveries from laterite tailings by organic and inorganic bio-acids, Hydrometallurgy, 2008, vol. 94, pp. 18–22.
Nayak, J.C., Some experiments on extraction of nickel and iron from nickeliferrous laterite deposits of sukinda valley, Trans. Indian Inst. Met., 1989, vol. 42, pp. 441–446.
Huamei Duan, Boron extraction from boron-concentrate ore by ammonium sulfate roasting method, J. Northeast. Univ. (Nat. Sci.), 2011, vol. 32, pp. 1724–1728.
Bing Chen, Xiaoyi Shen, Huimin Gu, Hongmei Shao, Yuchun Zhai, and Peihua Ma, Extracting reaction mechanism analysis of Zn and Si from zinc oxide ore by NaOH roasting method, J. Central South Univ., 2017, vol. 24, pp. 2266–2274.
Li, Q., Xu, D., Chen, Y., Yao, Y., Sun, Z., Ding, S., Decomposition of ammonium sulfate residue in a high solid/gas ratio suspension state with an industrial-scale reactor system(production line), Particuology, 2015, pp. 107–113.
Sadeghi, Nima, Moghaddam, J., and Ilkhch, M.O., Kinetics of zinc sulfide concentrate direct leaching in pilot plant scale and development of semi-empirical model, Trans. Nonfer. Met. Soc. China, 2017, p. 27.
Zhiqiang Ning, Yuchun Zhai, and Huamei Duan, Study on the dehydrate kinetics of bitter salt 7-hydrate, J. Chin. Soc. Rare Earths, 2010, vol. 28, p. 4.
Jin-Fang Lv, Yong-Xing Zheng, Xiong Tong, Yong-Ming Zheng, and Han-Ping Zhang, Mineralogy, physical characterization and magnetic separation performance of a raw ilmenite concentrate for its purification, Russ. J. Non-Ferrous Met., 2017, vol. 58 no. 2, pp. 101–108.
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by the National Basic Research Program of China, project no. 2014CB643405.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
About this article
Cite this article
Jie Li, Li, Y., Duan, H. et al. Experimental and Kinetic Study of Magnesium Extraction and Leaching from Laterite Nickel Ore by Roasting with Ammonium Sulfate. Russ. J. Non-ferrous Metals 59, 596–604 (2018). https://doi.org/10.3103/S1067821218060123
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821218060123