Abstract
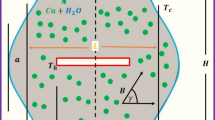
The effect of the electrolyte chemical composition and overheating on the size of a sideledge formed in an aluminum-smelting bath is investigated theoretically. Three electrolyte compositions are chosen: sodium cryolite with the cryolite ratio (CR) = 2.7, cryolite (CR) = 2.7 + 5 wt % CaF2, and cryolite (CR) = 2.7 + 5 wt % CaF2 + 5 wt % Al2O3. The electrolyte liquidus overheating temperatures are 5, 10, 15 and 20oC. The calculations are performed using the finite-element method. A simplified design of an aluminum cell with a prebaked anode is used. To calculate the temperature field, a mathematical model in the Boussinesq approximation is used. The model contains the Navier–Stokes equation, the thermal conductivity equation, and the incompressibility equation. The key role of electrolyte overheating on the sideledge formation is established. The resulting sideledge profile depends on the heat transfer coefficients and thermal properties of materials. The smallest sideledge thickness with the same electrolyte overheating is observed in cryolite with CR = 2.7, 5 wt % CaF2, and 5% by weight of Al2O3, and formed sideledge profiles for cryolite with KO = 2.7 and cryolite with KO = 2.7 and 5 wt % CaF2 almost coincide. The thickness of the sideledge formed with overheating of 5 K is from 7 cm or more, and the difference in temperature between the sideledge touching the electrolyte and airborne block wall is 20–25 K. Almost complete sideledge disappearance occurs when the electrolyte liquidus is overheated by 20 K.




Similar content being viewed by others
REFERENCES
Capitance, W. and Schmidt-Hatting, W., Magnetic fields in high amperage aluminum reduction cells, JOM, 1965, vol. 17, no. 3, pp. 271–275.
Zhou, J. and Dupuis, M., In-depth analysis of lining designs for several 420 kA electrolytic cells, Light Met, 2015, pp. 685–690.
Welch, B.J., Hyland, M.M., and James, B.J., Future materials requirements for the high-energy-intensity production of aluminum, JOM, 2001, vol. 53, no. 2, pp. 13–18.
Kvande, H., Bath chemistry and aluminum cell performance: facts, fictions, and doubts, JOM, 1994, vol. 46, no. 11, pp. 22–28.
Haupin, W., The influence of bath additives on Hall-Heroult bath properties, JOM, 1991, vol. 43, no. 11, pp. 28–34.
Dupuis, M., Computation of aluminum reduction cell energy balance using ANSYS ® Finite element models, TMS Light Met., 1998, pp. 409–417.
Beier, S., Chen, J., Fortin, H., and Fafard, M., FEM analysis of the anode connection in aluminum reduction cell, Light Met., 2011, pp. 979–984.
Wei, L., Jie, L., Yan-qing, L., and Ye-xiang, L., 2D Finite element analysis of thermal balance for drained aluminum reduction cells, J. Centr. South Univ. Technol., 2007, vol. 14, no. 6, pp. 783–787.
Dupius, M., Using ANSYS to model aluminum reduction cell since 1984 and beyond, in Proc. ANSYS Reg. Conf., Toronto, 2002.
Taylor, M., Etzion, R., Lavoie, P., and Tan, J.N., Energy balance regulation and flexible production: A new frontier for aluminum smelting, Metall. Mater. Trans. E, 2014, vol. 1, no. 4, pp. 292–302.
Lavoie, P., Namboothiri, S., Dorreen, M., Chen, J., Zeigler, D., and Taylor, M., Increasing the power modulation window of aluminium smelter pots with shell heat exchanger technology, Light Met., 2011, pp. 369–374.
Thonstad, J., Fellner, P., Haarberg, G.M., Hives, J., Kvande, H., and Sterten, A., Aluminium Electrolysis: Fundamentals of the Hall-Heroult Process, Dusseldorf: Aluminium-Verlag Market. Kommun., 2001, 3rd ed.
Solheim, A., Some aspects of heat transfer between bath and sideledge in aluminium reduction cells, Light Met., 2011, pp. 381–386.
Taylor, P. and Welch, B., Melt/freeze heat transfer measurements in cryolite-based electrolytes, Metall. Trans. B, 1987, vol. 18, no. 2, pp. 391–398.
Borisoglebskii, Yu.B., Galevskii, G.V., Kulagin, N.M., Mintsis, M.Ya., and Sirazutdinov, G.A., Metallurgiya aluminiya: Uchebnoe posobie (Aluminum Metallurgy: Textbook), Novosibirsk: Nauka, 1999.
Arkhipov, G.V., Pingin, V.V., Tretyakov, Y.A., and Polyakov, P.V., Simulation of cell thermoelectric field with consideration of electrochemical processes, Light Met., 2007, pp. 327–331.
Balkevich, V.L., Tehnicheskaya keramika: Uchebnik dlya vysshykh uchebnykh zavedenii (Technical Ceramics: Textbook for Higher Schools), Moscow: Stroiizdat, 1984.
ASM Metals Handbook. Vol. 1: Properties and Selection: Irons, Steels, and High-Performance Alloys, ASM, 1990, 10th ed.
Desai, P.D., Chu, T.K., James, H.M., and Ho, C.Y., Electrical resistivity of selected elements, J. Phys. Chem. Ref. Data, 1984, vol. 13, no. 4, pp. 1069–1096.
Shinno, H., Kitajima, M., and Okada, M., Thermal stress analysis of high heat flux materials, J. Nucl. Mater., 1988, vols. 155–157, pp. 290–294.
Giordanengo, B., Benazzi, N., Vinckel, J., Gasser, J.G., and Roubi, L., Thermal conductivity of liquid metals and metallic alloys, J. Non-Cryst. Solids, 2000, vols. 250–252, pp. 377–383.
Gale, W.F. and Totemeier, T.C., Smithells Metals Reference Book, Amsterdam: Elsevier, 1988.
Iida, T. and Guthrie, R.I.L., The Physical Properties of Liquid Metals, Oxford: Clarendon, 1988.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education and Science of the Russian Federation, agreement no. 14.607.21.0146; the unique agreement identifier is RFMEFI60716X0146.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by N. Korovin
About this article
Cite this article
Stakhanov, V.V., Redkin, A.A., Zaikov, Y.P. et al. Influence of Electrolyte Overheating and Composition on the Sideledge of an Aluminum Bath. Russ. J. Non-ferrous Metals 59, 471–475 (2018). https://doi.org/10.3103/S1067821218050188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821218050188