Abstract
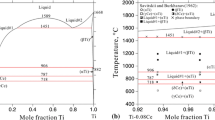
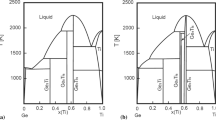
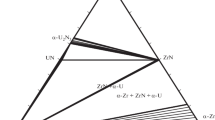
A model for calculation of three-phase equilibria with the participation of gas in binary systems is suggested and analyzed. This model is based on the joint solution of equations of the temperature and concentration dependences of the activity of components in solutions and liquidus equations in the T-x phase diagrams. Comparison calculated and experimental data by the location of the curves of three-phase equilibria α ⇆ L ⇆ G in the p-T phase diagrams of the Au-Cd and Cu-Zn systems confirmed the actuality of previous assumptions and the adequacy of the model suggested. The computing model is used to determine the parameters of extreme points in the curves of three-phase equilibria α ⇆ L ⇆ G of certain systems with the participation of metals.
Similar content being viewed by others
References
Rhines, F.N., Phase Diagrams in Metallurgy, New York: McGraw-Hill, 1956.
Levinskii, Yu.V., p-T-x diagrammy sostoyaniya dvukhkomponentnykh sistem (p-T-x Phase Diagrams of Binary Systems), Moscow: Metallurgiya, 1982.
Zlomanov, V.P., p-T-x diagrammy dvukhkomponentnykh sistem (p-T-x Diagrams of Binary Systems), Moscow: MGU, 1980.
Levinskii, Yu.V., p-T-x diagrammy sostoyaniya dvoinykh metallicheskikh sistem (p-T-x Phase Diagrams of Binary Metal Systems), Moscow: Metallurgiya, 1980, vol. 1.
Levinsky, Y., Pressure-Dependent Phase Diagrams of Binary Alloys, ASM International, Materials Park, 0H 44073, 1997, Bd. 1, 2.
Levinskii, Yu.V., Izv. Ross. Akad. Nauk, Metally, 2006, no. 6, p. 111.
Levinskii, Yu.V., Izv. Ross. Akad. Nauk, Metally, 2002, no. 5, p. 100.
Alcock, C.B., Itkin, V.P., and Horrigan, M.K., Can. Met. Quart., 1984, vol. 23, no. 3, p. 309.
Kowalski, M. and Spencer, P.J., J. Phase Equilib., 1993, vol. 14, no. 4, p. 432.
Okomoto, M. and Massalski, T.B., Bull. Alloy Phase Diagr., 1986, vol. 7, no. 1, p. 52.
Filby, J.D. and Pratt, N., Trans. Faraday Soc., 1964, vol. 60, p. 1934.
Bartha, L.J. and Aleksander, W.A., Can. J. Chem., 1965, vol. 43, p. 2319.
Everett, L.H. and Jacobs, P.W.M., Acta Metall., 1957, vol. 5, p. 1281.
Hargreaves, R., J. Inst. Metals, 1939, vol. 56, no. 1, p. 115.
Nemets, A.M. and Nikolaev, G.I., Zh. Prikl. Spektosk., 1971, vol. 15, no. 1, p. 724.
Schneider, A. and Schmidt, H., Z. Electrochem., 1942, Bd. 48, S. 627.
Kleppa, O.J., J. Phys. Chem., 1956, vol. 60, no. 7, p. 858.
Bonnier, E. and Durand, F., C. R. Acad. Sci., 1963, vol. 256, p. 1772.
Elliott, G.R.B., Herrick, C.C., Lemons, J.F., and Nordine, P.C., High Temp. Sci., 1969, vol. 1, p. 58.
Conant, D.R. and Swofford, H.S., J. Chem. Eng. Data, 1970, vol. 15, no. 5, p. 539.
Azakati, T. and Yazawa, A., J. Min. Metall. Inst. Jpn., 1968, vol. 84, p. 1663.
Solov’ev, S.P., Knyazev, M.V., Ivanov, Yu.I., and Vanyukov, A.V., Zavod. Lab., 1973, vol. 45, p. 841.
Spencer, P.Y., CALPHAD, 1986, vol. 10, no. 2, p. 175.
Sugino, S. and Hagiwara, H., J. Jap. Inst. Met., 1986, vol. 50, no. 2, p. 186.
Baker, E.H., Trans. Inst. Min. Met. C, 1970, vol. 79, C1–C5.
Brown, J.A. and Pratt, J.N., Metall. Trans. B, 1970, vol. 1, p. 2743.
Yazawa, A. and Gubcova, A., Trans. Jpn. Inst. Metals, 1970, vol. 11, p. 419.
Mozas, A. and Nerov, D., Arch. Eisenhüttenw, 1977, Bd. 48, S. 533.
Clougherty, E.V. and Kaufman, L., Acta Metall., 1963, vol. 11, p. 1043.
Chang, Y.A., Henning, G., and Naujock, D., Acta Metall., 1974, vol. 22, p. 7.
Grigorovich, K.V., Dashevskii, V.Ya., and Polyakov, Ya.Yu., Zh. Fiz. Khim., 1984, vol. 84, no. 4, p. 998.
Chen, Y.O. and Chang, Y.A., Met. Trans., 1978, vol. 98, p. 61.
Sharma, R.S. and Chang, Y.A., Z. Metallk., 1979, Bd. 70, S. 104.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.V. Levinskii, G.M. Vol’dman, N.V. Suchkova, D.A. Tkachuk, 2008, published in Izvestiya VUZ. Tsvetnaya Metallurgiya, 2008, No. 5, pp. 9–14.
About this article
Cite this article
Levinskii, Y.V., Vol’dman, G.M., Suchkova, N.V. et al. A procedure for calculation of three-phase equilibria with the participation of gas in binary systems. Russ. J. Non-ferrous Metals 49, 328–332 (2008). https://doi.org/10.3103/S1067821208050027
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821208050027