Abstract
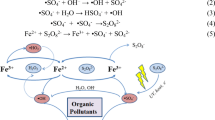
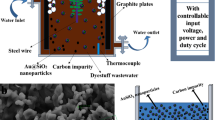
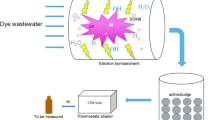
The plasma-chemical decomposition of Acid Violet 7 dye and simultaneous production of carbon micro- and nanomaterials were investigated. For the purification of wastewater contaminated with the dye, we used a plasma electrochemical setup with modified electrodes. The experiments were conducted in a galvanostatic mode with current intensities of 45 and 80 A and voltages ranging from 21 to 24 V. Two types of carbon-containing degradation products were formed. One type comprised fine-dispersed hydrophobic material, which, due to its low specific weight, hydrophobicity, and inclusion of gas bubbles, floated and concentrated at the interface between the solution and air phases. This material predominantly consisted of particles ranging in size from 1 to 5 µm, with some particles in the order of hundreds of nanometers present as well. Another type consisted of hydrophilic particles larger than 50 µm, which were deposited at the bottom of the cell. Plasma-chemical treatment leads to rapid degradation of the Acid Violet 7 molecule. Intensive discoloration of the dye solution occurs within the first 5–7 min. Exposure for 20 min resulted in an 8.4-fold decrease in chemical oxygen demand (COD).





Similar content being viewed by others
REFERENCES
Novoselov, K.S., Electric field effect in atomically thin carbon films, Science, 2004, vol. 306, no. 5696, pp. 666–669.
Albert, G., Nasibulin, Moisala, A., Jiang, H., and Kauppinen, E.I., Carbon nanotube synthesis from alcohols by a novel aerosol method, J. Nanopart. Res., 2006, vol. 8, pp. 465–475.
Mayne, M., Grobert, N., Terrones, M., Kamalakaran, R., Rühle, M., Kroto, H.W., and Walton, D.R.M., Pyrolytic production of aligned carbon nanotubes from homogeneously dispersed benzene-based aerosols, Chem. Phys. Lett., 2001, vol. 338, pp. 101–107.
Abdullayeva, S.H., Musayeva, N.N., Jabbarov, R.B., and Matsuda, T., Synthesis of carbon nanotubes from byproducts of oil refiner, World J. Condens. Matter Phys., 2014, vol. 4, no. 3, pp. 93–100.
Krause, B., Ritschel, M., Täschner, Ch., Oswald, S., Gruner, W., Leonhardt, A., and Pötschke, P., Comparison of nanotubes produced by fixed bed and aerosol-CVD methods and their electrical percolation behaviour in melt mixed polyamide 6.6 composites, Compos. Sci. Technol., 2010, vol. 70, pp. 151–160.
Goncharuk, V.V., Nauka o vode (Water Science), Kyiv: Naukova Dumka, 2010.
Ong, S.-T., Keng, P.-S., Lee, W.-N., Ha, S.-T., and Hung, Y.-T., Dye waste treatment, Water, 2011, vol. 3, pp. 157–176.
Chiang, L.-C., Chang, L.-E., and Tseng, S.-C., Electrochemical oxidation treatment of refractory organic pollutants, Water Sci. Technol., 1997, vol. 36, nos. 2–3, pp. 123–130. https://doi.org/10.2166/wst.1997.0499
Goncharuk, V.V., Klishchenko, R.E., and Kornienko, I.V., Destruction of non-ionic surfactant in plazma-chemical reactor, J. Water Chem. Technol., 2017, vol. 39, no. 6, pp. 355–359. https://doi.org/10.3103/S1063455X1706008X
Liu, Y., Chen, X., Li, J., and Burda, C., Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts, Chemosphere, 2005, vol. 61, no. 1, pp. 11–18.
So, C.M., Cheng, M.Y., Yu, J.C., and Wong, P.K., Degradation of azo dye Procion Red MX-5B by photocatalytic oxidation, Chemosphere, 2002, vol. 46, no. 6, pp. 905–912.
Maaß, S., Rojahn, J., Hänsch, R., and Kraume, M., Automated drop detection using image analysis for online particle size monitoring in multiphase systems, Comput. Chem. Eng., 2012, no. 45, pp. 27–37.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Klishchenko, R.E., Kornienko, I.V. & Honcharuk, V.V. Preparation of Carbon Micro- and Nanomaterials through Plasmochemical Treatment of Wastewater Contaminated with Acid Violet 7. J. Water Chem. Technol. 46, 169–175 (2024). https://doi.org/10.3103/S1063455X24020097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X24020097