Abstract
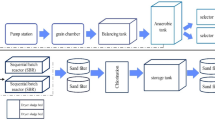
This study investigated water recovery with the treatment of leather industry processes wastewater (washing, pickling, and degreasing units) using coagulation, ultraviolet/persulfate (UV/PS) treatment, and nanofiltration processes. Coagulation studies were carried out using alum as the coagulant, and the highest chemical oxygen demand (COD) removal efficiency was obtained at pH 7 for all the wastewater. The highest COD and total organic carbon (TOC) removal were 80.9 and 50.5% in the wastewater washing unit (\({{{\text{S}}}_{{\text{2}}}}{\text{O}}_{8}^{{2 - }}\) : 8 g/L, pH 7) and 76.5 and 96.1% in the wastewater degreasing unit (\({{{\text{S}}}_{{\text{2}}}}{\text{O}}_{8}^{{2 - }}\) : 16 g/L, pH 6) using UV/PS oxidation, respectively. High COD and TOC removal could not be achieved with UV/PS oxidation in the wastewater pickling unit. In the studies performed with NP030 nanofiltration membrane after UV/PS oxidation, the highest permeability and COD removal was achieved at pH 7 under 4 × 105 Pa pressure in wastewater washing and degreasing units. After 75 min of nanofiltration at pH 7 in washing and degreasing units, the total filtrate amount was 39.8 and 42.3 L/m2 h, respectively. COD concentration in the wastewater washing unit decreased from 4434 to 138 mg/L, while it decreased from 5833 to 212 mg/L in the wastewater degreasing unit with coagulation, UV/PS processing, and nanofiltration. As a result, the treatment of leather industry wastewater through separate streams with coagulation, UV/PS, and nanofiltration, washing, and degreasing unit wastewater provides very high COD removal. Also, it has been shown impossible to treat the pickling unit wastewater by UV/PS oxidation.





Similar content being viewed by others
REFERENCES
Pal, P., Sardar, M., Pal, M., Chakrabortty, S., and Nayak, J., Modelling forward osmosis nanofiltration integrated process for treatment and recirculation of leather industry wastewater, Comput. Chem. Eng., 2019, vol. 127, pp. 99–110. https://doi.org/10.1016/j.compchemeng.2019.05.018
Mia, M.A.S., Alam, N.E., Ahmad, F., Alam, Z., and Rahman, M., Treatment of tannery wastewater by electrocougulation technology, J. Sci. Innovative Res., 2017, vol. 6, pp. 129–134. https://doi.org/10.31254/jsir.2017.6403
Thanikaivelan, P., Rao, J.R., Nair, B.U., and Ramasam, T., Recent trends in leather making: Processes, problems, and pathways, Crit. Rev. Environ. Sci. Technol., 2005, vol. 35, pp. 37–79. https://doi.org/10.1080/10643380590521436
Di Iaconi, C., Lopez, A., Ramadori, R., Di Pinto, A.C., and Passino, R., Combined chemical and biological degradation of tannery wastewater by a periodic submerged filter (SBBR), Water Res., 2002, vol. 36, pp. 2205–2214. https://doi.org/10.1016/S0043-1354(01)00445-6
Mandal, T., Dasgupta, D., Mandal, S., and Dafta, S., Treatment of leather industry wastewater by aerobic biological and Fenton oxidation process, J. Hazard. Mater., 2010, vol. 180, pp. 204–211. https://doi.org/10.1016/j.jhazmat.2010.04.014
Jazic, J.M., Durkic, T., Basic, B., Watson, M., Apostolovic, T., Tubic, A., and Agbaba, J., Degradation of a chloroacentanilide herbicide in natural waters using UV activated hydrogen peroxide, persulfate and peroxymonosulfate processes, Environ. Sci. Water Res. Technol., 2020, vol. 6, pp. 2800–2815. https://doi.org/10.1039/D0EW00358A
Liang, C., Wang, Z.S., and Bruel, C., Influence of pH on persulfate oxidation of TCE at ambient temperatures, Chemosphere, 2007, vol. 66, pp. 106–113. https://doi.org/10.1016/j.chemosphere.2006.05.026
Matzek, I.W. and Carter, K.E., Activated persulfate for organic chemical degradation: A review, Chemosphere, 2016, vol. 151, pp. 178–188. https://doi.org/10.1016/j.chemosphere.2016.02.055
Huang, Y.R., Kong, Y., Li, H.Z., and Wei, X.M., Removal of Crystal Violet by ultraviolet/persulfate: Effects, kinetics and degradation pathways, Environ. Technol. Innovation, 2020, vol. 18, p. 100780. https://doi.org/10.1016/j.eti.2020.100780
Crimi, M.L. and Taylor, J., Experimental evaluation of catalyzed hydrogen peroxide and sodium persulphate for destruction of BTEX contaminants, Soil Sediment Contam., 2007, vol. 16, pp. 29–45. https://doi.org/10.1080/15320380601077792
Ahmad, N.N.R., Ang, W.L., Teow, Y.H., Mohammad, A.W., and Hilal, N., Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: A review, J. Water Process Eng., 2022, vol. 45, p. 102478. https://doi.org/10.1016/j.jwpe.2021.102478
Shi, Y.T., Meng, X., Yao, L., and Tian, M., A full-scale study of nanofiltration: Separation and recovery of NaCl and Na2SO4 from coal chemical industry wastewater, Desalination, 2021, vol. 517, p. 115239. https://doi.org/10.1016/j.desal.2021.115239
Tian, D., Zhou, H., Zhang, H., Zhou, P., You, J., Yao, G., Pan, Z., Liu, Y., and Lai, B., Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies, Chem. Eng. J., 2022, vol. 428, p. 131166. https://doi.org/10.1016/j.cej.2021.131166
Chowdhury, M., Mostafa, M.G., Biswas, T.K., and Saha, A.K., Treatment of leather industrial effluents by filtration and coagulation processes, Water Resour. Ind., 2013, vol. 3, pp. 11–22. https://doi.org/10.1016/j.wri.2013.05.002
Al-Mutairi, N.Z., Hamoda, M.F., and Al-Ghusain, I., Coagulant selection and sludge conditioning in a slaughterhouse wastewater treatment plant, Bioresour. Technol., 2004, vol. 95, pp. 115–119. https://doi.org/10.1016/j.biortech.2004.02.017
Dalvand, A., Gholibegloo, E., Ganjali, M.R., Golchinpoor, N., Khazaei, M., Kamani, H., Hosseini, S.S., and Mahv, A.H., Comparison of Moringa stenopetala seed extract as a clean coagulant with alum and Moringa stenopetala–alum hybrid coagulant to remove direct dye from textile wastewater, Environ. Sci. Pollut. Res., 2016, vol. 23, pp. 16396–16405. https://doi.org/10.1007/s11356-016-6708-z
Wang, J. and Wang, S., Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants, Chem. Eng. J., 2018, vol. 334, pp. 1502–1517. https://doi.org/10.1016/j.cej.2017.11.059
Xinhui, X., Fengyi, Z., Jianju, L., Haizhou, Y., Liangliang, W., Qiaoyang, L., Junqiu, J., Guangshan, Z., and Qingliang, Z., A review study on sulfate radical based advanced oxidation processes for domestic/industrial wastewater treatment: Degradation, efficiency, and mechanism, Front. Chem., 2020, vol. 8, p. 592056. https://doi.org/10.3389/fchem.2020.592056
Chen, G., Wu, G., Li, N., Lu, X., Zhao, J., He, M., Yan, B., Zhang, H., Duan, X., and Wang, S., Landfill leachate treatment by persulphate related advanced oxidation technologies, J. Hazard. Mater., 2021, vol. 418, p. 126355. https://doi.org/10.1016/j.jhazmat.2021.126355
Gu, Z., Chen, W., Li, Q., and Zhang, A., Kinetics study of dinitrodiazophenol industrial wastewater treatment by a microwave coupled ferrous activated persulfate process, Chemosphere, 2019, vol. 215, pp. 82–91. https://doi.org/10.1016/j.chemosphere.2018.10.009
Chen, W., Luo, Y., Ran, G., and Li, Q., Microwave induced persulfate hydrogen peroxide binary oxidant process for the treatment of dinitrodiazophenol industrial wastewater, Chem. Eng. J., 2020, vol. 382, p. 122803. https://doi.org/10.1016/j.cej.2019.122803
Yang, S., Wang, P., Yang, X., Shan, L., Zhang, W., Shao, X., and Niu, R., Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV, and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide, J. Hazard. Mater., 2010, vol. 179, pp. 552–558. https://doi.org/10.1016/j.jhazmat.2010.03.039
Xu, C., Yang, G., Li, J., Zhang, S., Fang, Y., Peng, F., Zhang, S., and Qiu, R., Efficient purification of tetracycline wastewater by activated persulfate with heterogeneous Co–V bimetallic oxides, J. Colloid Interface Sci., 2022, vol. 619, pp. 188–197. https://doi.org/10.1016/j.jcis.2022.03.126
Anipsitakis, G.P. and Dionysiou, D.D., Radical generation by the interaction of transition metals with common oxidants, Environ. Sci. Technol., 2004, vol. 38, pp. 3705–3712. https://doi.org/10.1021/es035121o
Amor, C., Chueca, J.R., Joana, L., Fernandes, J.L., Dominguez, J.R., Lucas, M.J., and Peres, J.A., Winery wastewater treatment by sulphate radical based advanced oxidation processes (SR-AOP): Thermally vs UV-assisted persulphate activation, Process Saf. Environ. Prot., 2019, vol. 122, pp. 94–101. https://doi.org/10.1016/j.psep.2018.11.016
Michelon, M., Manera, A.P., Carvalho, A.L., and Filho, F.M., Concentration and purification of galacto-oligosaccharides using nanofiltration membranes, Int. J. Food Sci. Technol., 2014, vol. 49, pp. 1953–1961. https://doi.org/10.1111/ijfs.12582
Funding
This study was supported by the project no. NKUBAP.06.YL.21.336 within the scope of the master’s thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ali Rıza Dinçer, Çifçi, D.İ. & Karaca, F. Treatment of Leather Industry Wastewater Using Coagulation, Ultraviolet/Persulfate Processing and Nanofiltration for Water Recovery. J. Water Chem. Technol. 46, 176–185 (2024). https://doi.org/10.3103/S1063455X2402005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X2402005X