Abstract
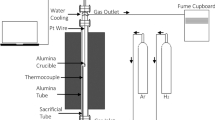
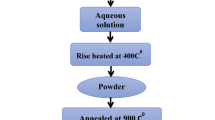
Lithium cobaltate (LiCoO2) was produced by carbon combustion synthesis of oxide (CCSO) using carbon nanoparticles as a fuel. In this method, the exothermic oxidation of carbon nanoparticles with an average size of 5 nm (specific surface 80 m2/g) gives rise to a self-propagating thermal wave with maximum temperatures of up to 900°C. The thermal front rapidly propagates through the mixture of solid reactants converting it to lithium cobaltate. XRD data suggest that the as-synthesized products were single phase. Carbon is not incorporated in the product and is evolved from the reaction zone as gaseous CO2. Thermogravimetric analysis was used to identify the features of interaction in the LiNO3-Co3O4-C system. The key features affecting the process-carbon pre-concentration in the reacting mixture and oxygen infiltration to the reaction zone-led to the formation of layered structure of LiCoO2 and affected the particle sizes. The synthesized crystalline nanoparticles were nearly spherical, and their average particle diameters ranged between 60 and 200 nm.
Similar content being viewed by others
References
Tarascon, J.M. and Armand, M., Issues and challenges facing rechargeable lithium batteries, Nature, 2001, vol. 414, pp. 359–367.
Balakrishnan, P.G., Ramesh, R., and Kumar, T.P., Safety mechanisms in lithium-ion batteries, J. Power Sources, 2006, vol. 155, no. 2, pp. 401–414.
Lithium ion batteries outlook and alternative energy vehicles (Hevs, Phevs, Evs)-technologies, Markets, Competitors and Opportunities: 2010–2020 Analysis and Forecasts, Rockville, MD; http://www.marketre-search.com/David-Company-v3832/Lithium-Ion-Batteries-Outlook-Alternative-6842261
Grosso, F., Cagnol, G.J., de Soler-Illia, A.A., Crepaldi, E.L.. Amenitsch, H., Brunet-Bruneau, A., Bourgeois, A., and Sanchez, C., Fundamentals of mesostructuring through evaporation-induced self-assembly, Adv. Funct. Mater., 2004, vol. 14, no. 4, pp. 309–322.
Zhong, Y.D., Zhao, X.B., and Cao, G.S., Characterization of solid-state synthesized pure and doped lithium nickel cobalt oxides, Mater. Sci. Eng., Ser. B, 2005, vol. 121, no. 3, pp. 248–254.
Julien, C., Local structure and electrochemistry of lithium cobalt oxides and their doped compounds, Solid State Ionics, 2003, vol. 157, nos. 1–4, pp. 57–71.
Fu, L.J., Liu, H., and Li, C., Electrode materials for lithium secondary batteries prepared by sol-gel methods, Prog. Mater. Sci., 2005, vol. 50, no. 7, pp. 881–928.
Li, G. and Zhang, J., Synthesis of nano-sized lithium cobalt oxide via a sol-gel method, Appl. Surf. Sci., 2012, vol. 258, no. 19, pp. 7612–7616.
Ying, T., Zhub, B., and Chen, Z., Synthesis mechanisms of lithium cobalt oxide prepared by hydrothermal-electrochemical method, J. Alloys Comp., 2007, vol. 430, nos. 1–2, pp. 222–225.
Kiran, K.B., Pollet, B.M., Artemenko, B.A., Miclau, A.M., and Grozescu, A.I., Synthesis of lithium cobalt oxide by single-step soft hydrothermal method, J. Solid State Chem., 2013, vol. 198, pp. 45–49.
Hobosyan, M.A., Kharatyan, S.L., Khachatryan, H.L., and Grigoryan, N.M., Combustion synthesis of lithium cobaltate, Int. J. Self-Prop. High-Temp. Synth., 2011, vol. 20, no. 2, pp. 107–112.
Martirosyan, K.S., and Luss, D., Carbon combustion synthesis of oxides, US Patent 7897135, 2011.
Merzhanov, A.G., The chemistry of self-propagating high temperature synthesis, J. Mater. Chem., 2004, vol. 14, no. 12, pp. 1779–1786.
Martirosyan, K.S. and Luss, D., Carbon combustion synthesis of oxides: Process demonstration and features, AIChE J., 2005, vol. 51, no. 10, pp. 2801–2810.
Markov, A., Filimonov, I., and Martirosyan, K.S., J. Comput. Phys., 2012, vol. 231, no. 20, pp. 6714–6724.
Martirosyan, K.S. and Luss, D., Carbon combustion synthesis of ferrites: Synthesis and characterization, Ind. Eng. Chem. Res., 2007, vol. 46, no. 5, pp. 1492–1499.
Martirosyan, K.S., Iliev, M., and Luss, D., Carbon combustion synthesis of nanostructured perovskites, Int. J. Self-Prop. High-Temp. Synth., 2007, vol. 16, no. 1, pp. 36–45.
Gan, Y., Zhang, L., Wen, Y., Wang, F., and Su, H., Carbon combustion synthesis of lithium cobalt oxide as cathode material for lithium ion battery, Particuology, 2008, vol. 6, no. 2, pp. 81–84.
Mamyrbaeva, Y., Hobosyan, M.A., Kumekov, S.E., and Martirosyan, K.S., Carbon combustion synthesis of lithium cobaltate, NSTI-NanoTech, 2013, vol. 2, pp. 657–659.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Mamyrbaeva, Y.Y., Hobosyan, M.A., Kumekov, S.E. et al. Electrochemical features of combustion-synthesized lithium cobaltate as cathode material for lithium ion battery. Int. J Self-Propag. High-Temp. Synth. 23, 1–8 (2014). https://doi.org/10.3103/S1061386214010087
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386214010087