Abstract
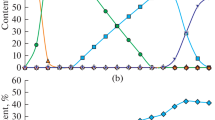
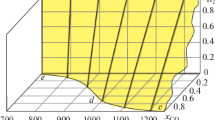
In this paper, we simulated the chemical process accompanied by the reduction of iron from hematite using the Terra computer program (the product from Bauman Moscow State Technical University). Carbon, hydrogen, and methane were taken as reducing agents. The equilibrium concentrations of the system components were determined by varying the consumption of reducing agents and the process temperature. The change in these concentrations at the boundaries of individual temperature regions was regarded as a result of the passage of corresponding chemical reactions in them. At the same time, it was noted that reactions of the non-variant type begin and end at the same fixed temperatures. Calculations showed that the Fe2O3 → Fe3O4 transformation in all cases was thermodynamically possible at temperatures exceeding 65°C. Consequently, it will be realized without complications at the furnace operating temperatures. The second stage of reduction also proceeded according to the unified scheme Fe3O4 → Fe, bypassing the participation of FeO oxide in it. The temperature of the beginning of the iron reduction by the C, H2, and CH4 components was 680, 350, and 520°C, respectively. In this case, only direct reduction of iron by the indicated components took place. An attempt to record the fact of indirect reduction using carbon monoxide as a reducing agent was unsuccessful even at its high consumption. Carbon monoxide decomposed at low temperatures according to the Boudouard-Bell reaction. Therefore, iron was reduced by “sooty” carbon, i.e., also by a direct method. At the final stage of the carbon-thermal process, depending on the composition of the system, the formation of iron carbide at 720°C can occur with its possible subsequent transformation back into iron, as well as the secondary oxidation of iron with the formation of wustite. Carbon dioxide takes an active part in these reactions. The reduction (or oxidative) efficiency of all elements and components of the Fe–O–C–H system is numerically estimated based on the calculation results of chemical processes at high temperatures. With a high degree of reliability, this allowed predicting the phase composition of the reaction products at the maximal process temperature (1500°C).


Similar content being viewed by others
REFERENCES
Voskoboinikov, V.G., Kudrin, V.A., and Yakushev, A.M., Obshchaya metallurgiya (General Metallurgy), Moscow: Akademkniga, 2005.
Vegman, E.F., Zherebin, B.N., Pokhvisnev, A.N., Yusfin, Yu.S., et al., Metallurgiya chuguna (Metallurgy of Cast Iron), Moscow: Akademkniga, 2004.
Pawlow, M.A., Metallurgie des Roheisens, Berlin: Verlag Technik, 1953, vol. 3.
Peacey, J.G. and Davenport, W.G., The Iron Blast Furnace: Theory and Practice, New York: Pergamon, 1979.
Sohn, H.Y., Sridhar, S., Aune, R.E., et al., Fundamentals of Metallurgy, Cambridge: Woodhead, 2005.
Zhou, X.L. and Du, Z.N., The introduction of COREX process development, Adv. Mater. Res., 2013, vols. 774–776, pp. 1430–1433.
Mouer, A., et al., The Lion Group & MIDREX Experience: Operational Aspects of Lion’s MIDREX HDRI/HBI Plant, Charlotte, NC: MIDREX, 2009, pp. 3–7.
Unal, H., Turgut, E., Atapek, S., and Alkan, A., Direct reduction of ferrous oxides to form an iron-rich alternative charge material, High Temp. Mater. Process., 2015, vol. 34, no. 8, pp. 751–756.
Jones, W.D., Fundamental Principles of Powder Metallurgy, London: Edward Arnold, 1960.
Zel’dovich, Ya.B., Proof of the uniqueness of the solution to the equations of the mass law of action, Zh. Fiz. Khim., 1938, vol. 11, no. 5, pp. 685–687.
Brinkley, S.R., Calculation of equilibrium composition of systems of many constituents, J. Chem. Phys., 1947, vol. 15, no. 2, pp. 107–110.
Berdnikov, V.I., Computer calculation of chemical equilibrium in multicomponent systems, Izv. Vyssh. Uchebn. Zaved., Chern. Metall., 1984, no. 4, pp. 120–122.
Gurvich, L.V., Veitz, I.V., et al., Thermodynamic Properties of Individual Substances: Methods and Computation, Boca Raton, FL: CRC Press, 1989, 4th ed.
White, W.B., Johnson, S.M., and Dantzig, G.B., Chemical equilibrium in complex mixtures, J. Chem. Phys., 1958, vol. 28, no. 5, pp. 751–755.
Trusov, B.G., Baza dannykh i programmnyi kompleks Terra, redaktsiya 6.3 (Terra Database and Software, Version 6.3), Moscow: Mosk. Gos. Tekh. Univ. im. N.E. Baumana, 2013.
Gurvich, L.V., Iorish, V.S., et al., IVTANTHERMO—A Thermodynamic Database and Software System for the Personal Computer: User’s Guide, Boca Raton, FL: CRC Press, 1993.
Berdnikov, V.I., Adaptation of the balance thermodynamic analysis to the mechanism of silicon reduction in a ferroalloy furnace, Stal’, 1991, no. 2, pp. 42–45.
Berdnikov, V.I., Gudim, Yu.A., and Karteleva, M.I., The phase rule in the analysis of metallurgical processes, Steel Transl., 2010, vol. 40, no. 12, pp. 1025–1028.
Mikhailov, G.G., Kuznetsov, Yu.S., Kachurina, O.I., and Chrnukha, A.S., Analysis of phase equilibria in the iron oxides-carbon-CO-CO2 system, Vestn. Yuzh.-Ural. Gos. Univ., Ser. Metall., 2013, vol. 13, no. 1, pp. 6–13.
Tret’yakov, V.D. and Putlyaev, V.I., Vvedenie v Khimiya tverdofaznykh materialov (Introduction into Chemistry of Solid Phase Materials), Moscow: Nauka, 2006.
Kuznetsov, Yu.S. and Kachurina, O.I., Redox properties of gas phases, Steel Transl., 2018, vol. 48, no. 1, pp. 25–33.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Ivanov
About this article
Cite this article
Berdnikov, V.I., Gudim, Y.A. Chemical Reactions in the Reduction of Iron from Oxides. Steel Transl. 50, 773–778 (2020). https://doi.org/10.3103/S0967091220110042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091220110042