Abstract
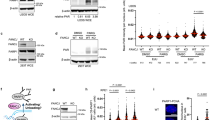
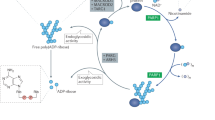
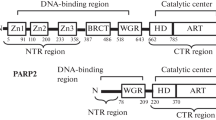
Poly ADP-ribose Polymerase 1 (PARP1) is an important enzyme that is involved in DNA repair, replication, and transcription. Prospective anticancer drug gossypol inhibits human PARP1, but the mechanism of inhibition remains unknown. It has been shown previously that gossypol interacts with purified BRCA1 C-terminus (BRCT) domain in vitro. However, it remained unclear whether gossypol inhibits PARP1 through the BRCT domain in the context of full-length protein. Here, we report that the BRCT domain within the full-length PARP1 protein is not required for the inhibition of catalytic activity of PARP1 by gossypol. Our results, obtained using a series of PARP1 mutations and H4-dependent pathway of PARP1 activation, also show that none of the zinc fingers or other DNA binding domains of PARP1 are involved in the inhibition of the PARP1 catalytic activity by gossypol. Thus, the likely candidate target(s) for gossypol action are the other domains of PARP1, or the interdomain linkers.
Similar content being viewed by others
References
Ame, J.C., Spenlehauer, C., and De Murcia, G., The PARP superfamily, BioEssays, 2004, vol. 26, no. 8, pp. 882–893.
Ludwig, A., Behnke, B., Holtlund, J., and Hilz, H., Immunoquantitation and size determination of intrinsic poly(ADP-ribose) polymerase from acid precipitates. An analysis of the in vivo status in mammalian species and in lower eukaryotes, J. Biol. Chem., 1988, vol. 263, no. 15, pp. 6993–6999.
Yamanaka, H., Penning, C.A., Willis, E.H., Wasson, D.B., and Carson, D.A., Characterization of human poly(ADP-ribose) polymerase with autoantibodies, J. Biol. Chem., 1988, vol. 263, no. 8, pp. 3879–3883.
Haince, J.F., McDonald, D., Rodrigue, A., Dery, U., Masson, J.Y., Hendzel, M.J., and Poirier, G.G., PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites, J. Biol. Chem., 2008, vol. 283, no. 2, pp. 1197–1208.
Thomas, C. and Tulin, A.V., Poly-ADP-ribose polymerase: Machinery for nuclear processes, Mol. Aspects Med., 2013, vol. 34, no. 6, pp. 1124–1137.
Nishikimi, M., Ogasawara, K., Kameshita, I., Taniguchi, T., and Shizuta, Y., Poly(ADP-ribose) synthetase. The DNA binding domain and the automodification domain, J. Biol. Chem., 1982, vol. 257, no. 11, pp. 6102–6105.
Kameshita, I., Matsuda, Z., Taniguchi, T., and Shizuta, Y., Poly (ADP-ribose) synthetase. Separation and identification of three proteolytic fragments as the substrate-binding domain, the DNA-binding domain, and the automodification domain, J. Biol. Chem., 1984, vol. 259, no. 8, pp. 4770–4776.
Gibson, B.A. and Kraus, W.L., New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs, Nat. Rev. Mol. Cell Biol., 2012, vol. 13, no. 7, pp. 411–424.
Langelier, M.F., Servent, K.M., Rogers, E.E., and Pascal, J.M., A third zinc-binding domain of human poly(ADP-ribose) polymerase-1 coordinates DNAdependent enzyme activation, J. Biol. Chem., 2008, vol. 283, no. 7, pp. 4105–4114.
Tao, Z., Gao, P., Hoffman, D.W., and Liu, H.W., Domain C of human poly(ADP-ribose) polymerase-1 is important for enzyme activity and contains a novel zinc-ribbon motif, Biochemistry, 2008, vol. 47, no. 21, pp. 5804–5813.
Langelier, M., Ruhl, D.D., Planck, J.L., Kraus, W.L., and Pascal, J.M., The Zn3 domain of human poly(ADP-ribose) polymerase-1 (PARP1) functions in both DNA-dependent poly(ADP-ribose) synthesis activity and chromatin compaction, J. Biol. Chem., vol. 285, no. 24, pp. 18877–18887.
Langelier, M.F., Planck, J.L., Roy, S., and Pascal, J.M., Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1, Science, 2012, vol. 336, no. 6082, pp. 728–732.
Bork, P., Hofman, K., Buche, P., Neuwal, A.F., Altschu, S.F., and Koonin, E.V., A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins, FASEB J., 1997, vol. 11, no. 1, pp. 68–76.
Masson, M., Niedergang, C., Schreiber, V., Muller, S., Menissier-de Murcia J., and De Murcia, G., XRCC1 is specifically associated with poly(ADPribose) polymerase and negatively regulates its activity following DNA damage, Mol. Cell. Biol., 1998, vol. 18, no. 6, pp. 3563–3571.
Masson, M., Murcia, J., Mattei, M.G., De Murcia, G., and Niedergang, C.P., Poly(ADPribose) polymerase interacts with a novel human ubiquitin conjugating enzyme: hUbc9, Gene, 1997, vol. 190, no. 2, pp. 287–296.
Buki, K.G., Bauer, P.I., Hakam, A., and Kun, E., Identification of domains of poly(ADP-ribose) polymerase for protein binding and selfassociation, J. Biol. Chem., 1995, vol. 270, no. 7, pp. 3370–3377.
Nie, J., Sakamoto, S., Song, D., Qu, Z., Ota, K., and Taniguchi, T., Interaction of Oct-1 and automodification domain of poly(ADP-ribose) synthetase, FEBS Lett., 1998, vol. 424, nos. 1–2, pp. 27–32.
Griesenbeck, J., Ziegler, M., Tomilin, N., Schweiger, M., and Oei, S.L., Stimulation of the catalytic activity of poly(ADP-ribosyl) transferase by transcription factor Yin Yang 1, FEBS Lett., 1999, vol. 443, no. 1, pp. 20–24.
Na, Z., Peng, B., Ng, S., Pan, S., Lee, J.S., Shen, H.M., and Yao, S.Q., A small-molecule protein-protein interaction inhibitor of PARP1 that targets its BRCT domain, Angew. Chem., Int. Ed. Engl., 2015, vol. 54, no. 8, pp. 2515–2519.
Malyuchenko, N.V., Kotova, E.Yu., Kulaeva, O.I., Kirpichnikov, M.P., and Studitskiy, V.M., PARP1 inhibitors: Antitumor drug design, Acta Naturae, 2015, vol. 7, no. 3, pp. 27–37.
Gilbert, N.E.O., Reilly, J.E., Chang, C.J., Lin, Y.C., and Brueggemeier, R.W., Antiproliferative activity of gossypol and gossypolone on human breast cancer cells, Life Sci., 1995, vol. 57, no. 1, pp. 61–67.
Langelier, M.F., Planck, J.L., Servent, K.M., and Pascal, J.M., Purification of human PARPI and PARPI domains from E. coli for structural and biochemical analysis, Methods Mol. Biol., 2011, vol. 780, pp. 209–226.
Kotova, E., Pinnola, A.D., and Tulin, A.V., Smallmolecule collection and high-throughput colorimetric assay to identify PARP-1 inhibitors, Methods Mol. Biol., 2011, vol. 780, pp. 491–516.
Dawicki-McKenna, J.M., Langelier, M.F., DeNizio, J.E., Riccio, A.A., Cao, C.D., Karch, K.R., McCauley, M., Steffen, J.D., Black, B.E., and Pascal, J.M., PARP-1 activation requires local unfolding of an autoinhibitory domain, Mol. Cell, 2015, vol. 60, no. 5, pp. 755–768.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S. Gross, E.Yu. Kotova, N.V. Maluchenko, J.M. Pascal, V.M. Studitsky, 2016, published in Vestnik Moskovskogo Universiteta, Seriya 16: Biologiya, 2016, No. 4, pp. 61–65.
About this article
Cite this article
Gross, S., Kotova, E.Y., Maluchenko, N.V. et al. Evaluating Parp1 domains as gossypol targets. Moscow Univ. Biol.Sci. Bull. 71, 235–239 (2016). https://doi.org/10.3103/S0096392516040106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0096392516040106