Abstract
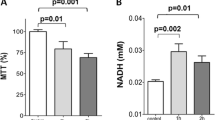
Oxidative stress is an important pathophysiological factor in chronic respiratory diseases. Our study aimed at elucidating through which pathway oxidative stress–mediated apoptosis occurs at the gene expression level under oxidative stress in the human bronchial epithelial cell line BEAS-2B. Suitable doses and time period were detected by exposing BEAS-2B cells to hydrogen peroxide (H2O2) at different doses and time periods, and the oxidative-damaged cell culture model was designed. The treatment and control groups were compared in terms of gene expression levels determined by Quantitative Real Time Polymerase Chain Reaction. The oxidative-damaged cell model was confirmed by the spectrophotometric measurement of malondialdehyde and catalase activity (p < 0.05). Caspase-3, caspase-9, bax, and bak gene expression levels increased significantly in the treatment groups compared to the control group (p < 0.05). There were not any significant differences between the groups in terms of caspase-8, Bcl-2, and bik (p > 0.05). p53 and p21 gene expression levels were found to be significantly higher in the treatment groups (p < 0.05). H2O2-induced oxidative stress, induced apoptosis through the intrinsic pathway at gene expression level in the bronchial epithelial BEAS-2B cells was observed.






Similar content being viewed by others
REFERENCES
Aebi, H., Catalase in vitro, Methods Enzymol., 1984, vol 105, pp. 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Aebi, H., Wyss, S.R., Scherz, B., et al., Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits, Eur. J. Biochem., 1974, vol. 48, no. 1, pp. 137–145. https://doi.org/10.1111/j.1432-1033.1974.tb03751.x
Antognelli, C., Gambelunghe, A., Talesa, V.N., et al., Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: involvement of superoxide anion, hydrogen peroxide and NF-kappaB, Apoptosis, 2014, vol. 19, no. 1, pp. 102–116. https://doi.org/10.1007/s10495-013-0902-y
Begnini, K.R., Moura de Leon, P.M., Thurow, H., et al., Brazilian red propolis induces apoptosis-like cell death and decreases migration potential in bladder cancer cells, Evid. Based Complement Alternat. Med., 2014, vol. 2014, article 639856. https://doi.org/10.1155/2014/639856
Chen, J.J., Bertrand, H., and Yu, B.P., Inhibition of adenine nucleotide translocator by lipid peroxidation products, Free Radic. Biol. Med., 1995, vol. 19, no. 5, pp. 583–590. https://doi.org/10.1016/0891-5849(95)00066-7
Cho, I.H., Gong, J.H., Kang, M.K., et al., Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling, BMC Pulm. Med., 2014, vol. 14, p. 122. https://doi.org/10.1186/1471-2466-14-122
Downs, C.A., Montgomery, D.W., and Merkle, C.J., Age-related differences in cigarette smoke extract-induced H2O2 production by lung endothelial cells, Microvasc. Res., 2011, vol. 82, no. 3, pp. 311–317. https://doi.org/10.1016/j.mvr.2011.09.013
Gallet, P.F., Petit, J.M., Maftah, A., et al., Asymmetrical distribution of cardiolipin in yeast inner mitochondrial membrane triggered by carbon catabolite repression, Biochem. J., 1997, vol. 324 (Pt. 2), pp. 627–634. https://doi.org/10.1042/bj3240627
Gurr, J.R., Wang, A.S., Chen, C.H., et al., Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells, Toxicology, 2005, vol. 213, nos. 1–2, pp. 66–73. https://doi.org/10.1016/j.tox.2005.05.007
Hsia, T.C. and Yin, M.C., S-Ethyl cysteine and S-methyl cysteine protect human bronchial epithelial cells against hydrogen peroxide induced injury, J. Food Sci., 2015, vol. 80, no. 9, pp. H2094–H2101. https://doi.org/10.1111/1750-3841.12973
Huang, Y.D., Li, P., Tong, X., et al., Effects of bleomycin A5 on caspase-3, P53, bcl-2 expression and telomerase activity in vascular endothelial cells, Indian J. Pharmacol., 2015, vol. 47, no. 1, pp. 55–58. https://doi.org/10.4103/0253-7613.150337
Jain, S.K., Membrane lipid peroxidation in erythrocytes of the newborn, Clin. Chim. Acta, 1986, vol. 161, no. 3, pp. 301–306. https://doi.org/10.1016/0009-8981(86)90014-8
Kocabaş, A., Kronik obstrüktif akciğer hastaliği epidemiyolojisi ve risk faktörleri, TTD Toraks Cerrahisi Bülteni, 2010, vol. 1, no. 2, pp. 105–113.
Lartillot, S., Kedziora, P., and Athias, A., Purification and characterization of a new fungal catalase, Prep. Biochem., 1988, vol. 18, no. 3, pp. 241–246. https://doi.org/10.1080/00327488808062526
Lu, Y., Xu, D., Zhou, J., et al., Differential responses to genotoxic agents between induced pluripotent stem cells and tumor cell lines, J. Hematol. Oncol., 2013, vol. 6, no. 1, p. 71. https://doi.org/10.1186/1756-8722-6-71
Martins, D. and English, A.M., Catalase activity is stimulated by H2O2 in rich culture medium and is required for H2O2 resistance and adaptation in yeast, Redox Biol., 2014, vol. 2, pp. 308–313. https://doi.org/10.1016/j.redox.2013.12.019
Mosmann, T., Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, vol. 65, nos. 1–2, pp. 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Nomura, K., Imai, H., Koumura, T., et al., Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis, Biochem. J., 2000, vol. 351 (Pt. 1), pp. 183–193. https://doi.org/10.1042/0264-6021:3510183
Öner Erkekol, F, Köktürk, N., Mungan, D., et al., Türkiye kronik hava yolu hastalıkları önleme ve kontrol programı (GARD Türkiye) birinci basamakta çalışan hekim eğitimi bilgi değerlendirme sonuçları, Tuberk. Toraks, 2017, vol. 65, no. 2, pp. 80–89. https://doi.org/10.5578/tt.53804
Orrenius, S., Reactive oxygen species in mitochondria-mediated cell death, Drug Metab. Rev., 2007, vol. 39, nos. 2–3, pp. 443–455. https://doi.org/10.1080/03602530701468516
Pisoschi, A.M. and Pop, A., The role of antioxidants in the chemistry of oxidative stress: a review, Eur. J. Med. Chem., 2015, vol. 97, pp. 55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Poljsak, B., Suput, D., and Milisav, I., Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants, Oxid. Med. Cell Longev., 2013, vol. 2013, article 956792. https://doi.org/10.1155/2013/956792
Shidoji, Y., Hayashi, K., Komura, S., et al., Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation, Biochem. Biophys. Res. Commun., 1999, vol. 264, no. 2, pp. 343–347. https://doi.org/10.1006/bbrc.1999.1410
Tsao, S.M. and Yin, M.C., Antioxidative and antiinflammatory activities of asiatic acid, glycyrrhizic acid, and oleanolic acid in human bronchial epithelial cells, J. Agric. Food Chem., 2015, vol. 63, no. 12, pp. 3196–3204. https://doi.org/10.1021/acs.jafc.5b00102
Tseng, C.Y., Wang, J.S., Chang, Y.J., et al., Exposure to high-dose diesel exhaust particles induces intracellular oxidative stress and causes endothelial apoptosis in cultured in vitro capillary tube cells, Cardiovasc. Toxicol., 2015, vol. 15, no. 4, pp. 345–354. https://doi.org/10.1007/s12012-014-9302-y
Ushmorov, A., Ratter, F., Lehmann, V., et al., Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome c release, Blood, 1999, vol. 93, no. 7, pp. 2342–2352
Wu, J., Shi, Y., Asweto, C.O., et al., Fine particle matters induce DNA damage and G2/M cell cycle arrest in human bronchial epithelial BEAS-2B cells, Environ. Sci. Pollut. Res. Int., 2017, vol. 24, no. 32, pp. 25071–25081. https://doi.org/10.1007/s11356-0170090-3
Wu, X.F., Wang, L.Y., Yi, J.H., et al., Protective effect of paeoniflorin against PM2.5-induced damage in BEAS-2B cells, Nan Fang Yi Ke Da Xue Xue Bao, 2018, vol. 38, no. 2, pp. 168–173. https://doi.org/10.3969/j.issn.1673-4254.2018.02.08
Yarosz, E.L. and Chang, C.H., The role of reactive oxygen species in regulating T cell- mediated immunity and disease, Immune Network, 2018, vol. 18, no. 1, e14. https://doi.org/10.4110/in.2018.18.e14
Yi, S., Zhang, F., Qu, F., et al., Water-insoluble fraction of airborne particulate matter (PM10) induces oxidative stress in human lung epithelial A549 cells, Environ. Toxicol., 2014, vol. 29, no. 2, pp. 226–233. https://doi.org/10.1002/tox.21750
ACKNOWLEDGMENTS
Meral Urhan-Kucuk and Hasret Ecevit have carried out the design and coordinated the study as well as they have checked the last revisions and send the manuscript. The author of the PhD thesis Hasret Ecevit has carried out the experimental analysis and prepared the manuscript. Abdullah Arpacı has evaluated the experimental analysis. Haluk Uluca and Duygu Tap have assisted in experimental analysis.
Funding
This study is supported by Hatay Mustafa Kemal University Scientific Research Projects Unit (Project no. 16745) Hatay, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
About this article
Cite this article
Ecevit, H., Urhan-Kucuk, M., Uluca, H. et al. The Role of Oxidative Stress in Apoptosis and Cell Proliferation of Human Bronchial Epithelial Cells. Cytol. Genet. 55, 283–289 (2021). https://doi.org/10.3103/S0095452721030026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721030026