Abstract
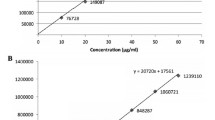
A procedure was proposed for quantitative analysis of Codelac Broncho tablets and syrup, a new original drug, by high-pressure liquid chromatography. The active principles of the tablets were separated in 6 min with an efficient resolution of all component peaks. Preproduction tablet samples were analyzed. The results of the analysis meet the requirements of normative technical documentation and technologic loads. The adequacy of the results was validated by the analysis of model solutions that contained all active principles and adjuvants. For the syrup, two versions of analysis were proposed, in isocratic and gradient elution modes. These versions are virtually equivalent with respect to the analysis time and precision. The isocratic elution version is, however, easier to implement and is proposed for inclusion into the draft pharmacopoeic standards for commercial production.
Similar content being viewed by others
References
Schoeunmakers, P.J., The Optimization of Chromatographic Selectivity: A Guide to Method Development, Amsterdam: Elsevier, 1986. Translated under the title Optimizatsiya selektivnosti v khromatografii, Moscow, 1989.
Golubitskii, G.B., Budko, E.V., Prokhoda, E.F., Pokrovskii, M.V., and Zakharova, E.V., RF Patent no. 2267115.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.B. Golubitskii, V.M. Ivanov, 2007, published in Vestnik Moskovskogo Universiteta. Khimiya, 2007, No. 6, pp. 395–400.
About this article
Cite this article
Golubitskii, G.B., Ivanov, V.M. Quantitative analysis of Codelac Broncho tablets and syrup by high-pressure liquid chromatography. Moscow Univ. Chem. Bull. 62, 325–329 (2007). https://doi.org/10.3103/S0027131407060065
Received:
Issue Date:
DOI: https://doi.org/10.3103/S0027131407060065