Abstract
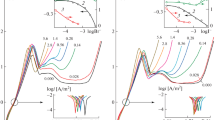
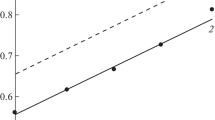
The influence of domestic nitrogen-containing corrosion inhibitors of the VNKh-L type on corrosion regularities of a zinc coating on steel in neutral media is investigated. This work is aimed at studying the surface structure of a corroding zinc coating, as well as the influence of conditions simulating the degradation of inhibitors during real operation on their protective properties. Mechanical activation in a ball planetary mill is used to simulate the deformation and thermal operational conditions of inhibitors. Corrosion of a zinc coating on steel is carried out in a sulfate–chloride medium simulating atmospheric corrosion and in a borate buffer solution. The concentration of inhibitors in the corrosion media was 0.2 wt %. The morphology of a corroded surface of a zinc coating is studied using a Philips SEM-515 scanning electron microscope (at an accelerating voltage of 10 kV) with an X-ray microprobe. Studies of the corrosion rate of zinc coating on St 08 are carried out by the indirect measurement of corrosion resistance with the help of a MONIKOR-1 corrosion meter. A borate buffer solution (Na2B4O7 + H3BO3, pH 6.6) and a solution simulating atmospheric corrosion (NaCl + Na2SO4, pH 6.0) are used as corrosion media. The corrosion rate of samples in corrosive media without an inhibitor is accepted 1. The exposure time of each sample in corrosive media is 3 h. The chemical composition of corrosion products is studied by the mirror reflection in the IR range. The IR spectra of the metal plate surface are recorded using an FSM-1202 IR Fourier spectrometer in a wavelength range of 450–4000 cm–1 with a resolution of 2 cm–1 and an accumulation of 100 scans. To record the reflection spectra, a mirror-reflection attachment with a 10° angle of incidence is used. The corrosion rate of zinc coating in sulfate–chloride and solvent media in the presence of inhibitors based on benzotriazole and cyclohexylamine is practically not decreased when compared with the corrosion rate in the same media without inhibitors. The addition of both initial and mechanically activated inhibitors based on morpholine and benzotriazole to the corrosion medium decreases the corrosion rate of iron when compared with the corrosion in the same media without inhibitors. The pitting corrosion of a zinc coating is observed in the presence of initial and mechanically activated inhibitors of both types in studied corrosion media. Herewith, the pitting depth is smaller than the zinc-coating thickness under these conditions.











Similar content being viewed by others
REFERENCES
Leygraf, C. and Graedel, T., Atmospheric Corrosion, New Haven: Wiley, 2016.
Sastri, V.S., Ghali, E., and Elboujdaini, M., Practical solutions, Corrosion Prevention and Protection, England: Wiley, 2012, pp. 461–551.
Thomas, S., Birbilis, N., Venkatraman, M.S., and Cole, I.S., Corrosion of zinc as a function of pH, Corrosion, 2012, vol. 68, no. 1, pp. 015009-1–015009-9.
Desai, M.N., Rana, S.S., and Gandhi, M.H., Corrosion inhibitors for zinc, AntiCorros. Meth. Mater., 1973, vol. 20, no. 12, pp. 3–6. https://doi.org/10.1108/eb006939
Aramaki, K., Effects of organic inhibitors on corrosion of zinc in an aerated 0.5 M NaCl solution, Corros. Sci., 2001, vol. 43, no. 10, pp. 1985–2000.
Wang, K., Pickering, H.W., and Weil, K.G., Corrosion inhibition of zinc by benzotriazole with electrochemical quartz crystal microbalance, J. Electrochem. Sci., 2003, vol. 150, no. 41, pp. 176–180.
Ekimik, V.V., Berezhnaya, A.G., and Svyataya, M.K., Inhibition of dissolution stages of zinc by some acridines, Zashch. Met., 2001, no. 6, pp. 589–591.
Ličina, S., Ostojič, J., Gutič, S., and Cacan, M., Influence of chloride ions on various corrosion resistance of zinc coating, Bull. Chem. Technol. Bosnia Herzeg., 2015, no. 44, pp. 33–38.
Mani, N., Iyer Venkatakrishna S., and Bahadur Lai, Influence of anions on the inhibition of corrosion of zinc in acidic solutions by N-heterocylics, Bull. Electrochem., 2003, vol. 19, no. 21, pp. 53–60.
Mani, N., Iyer Venkatakrishna S., and Bahadur Lai, Pyridine and its derivatives as inhibitors for the corrosion of zinc in acidic solutions, J. Electrochem. Soc. India, 2003, vol. 52, no. 1, pp. 23–28.
Fattah, A.A.A., Mabrouk, E.M., Elgalil, R.M.A., and Ghoneim, M.M., N-heterocyclic compounds as corrosion inhibitors for Zn in HCl acid solutions, Bull. Soc. Chim. Fr., 1991, vol. 1, pp. 48–53.
Wippermann, K., Schaltze, J.W., Kessel, R., and Penninger, T., The inhibition of zinc corrosion by bisaminotriazole and other triazole derivatives, Corros. Sci., 1991, vol. 32, pp. 205–223.
Muller, B. and Imblo, G., Heterocycles as corrosion inhibitors for zinc pigments in aqueous alkaline media, Corros. Sci., 1996, vol. 38, pp. 293–300.
Lomovskii, O.I. and Boldyrev, V.V., Mekhanokhimiya v reshenii ekologicheskikh zadach: analiticheskii obzor. Ser. Ekologiya (Mechanochemistry in Solving Environmental Problems: Analytical Review. Ser. Ecology), Novosibirsk: GPNTB SO RAN, 2006, vyp. 79.
Cleramunt, R.M., Lopez, C., Sanz, D., and Elguero, J., Mechano heterocyclic chemistry: grinding and ball milling, Adv. Hetr. Chem., 2014, vol. 112, pp. 117–143.
Shakhtshneider, T.P., Influence of mechanical treatment of physicochemical properties of medicines, Doctoral (Chem.) Dissertation, Novosibirsk: IKhTTM SO RAN, 2013.
Alekseev, V.V., Optical isomerism and pharmacological activity of medicinal preparations, Soros. Obraz. Zh., 1998, no. 1, pp. 49–55.
Kanunnikova, O.M., Aksenova, V.V., Karban, O.V., Muhgalin, V.V., Senkovski, B.V., and Ladjanov, V.I., Mechanical activation effect on structure, physicochemical, and biological properties of potassium orotate/magnesium orotates, IOP Conf. Ser.: Mater. Sci. Eng., 2017, vol. 283, p. 012004.
Reshetnikov, S.M., Ingibitory kislotnoi korrozii metallov (Inhibitors of Acidic Metal Corrosion), Leningrad: Khimiya, 1986.
Pletnev, M.A., Reshetnikov, S.M., Ternavtseva, I.V., Shabanova, I.N, Ponamareva, I.L., and Dobysheva, L.V., Features of inhibitory action of quaternary ammonium salts, phosphonium and arsonium during corrosion of iron in sulfuric acid, Zashch. Met., 1990, vol. 26, no. 1, pp. 144–147.
Reshetnikov, S.M., Pletnev, M.A., and Makarova, L.L., Features of the influence of quaternary ammonium salts on the anodic dissolution of iron in hydrochloric acid, in Tezisy 9-go Vsesoyuznogo simpoziuma “Dvoinoi sloi i adsorbtsiya na tverdykh elektrodakh” (Abstracts of 9th All-Union Symp. “Double Layer and Adsorption on Solid Electrodes”), Tartu: Tartuskii Gos. Univ., 1991, pp. 152–154.
Pletnev, M.A., Shirobokov, I.B., Butolina, O.V., and Reshetnikov, S.M., Influence of tetralkylammonium salts on the electrical conductivity of acidic bromide solutions, Elektrokhimiya, 1993, vol. 29, pp. 1137–1145.
Shirobokov, I.B., Korepanova, T.A., Pletnev, M.A., and Reshetnikov, S.M., Influence of inorganic and organic cations on the conductivity of acidic bromide solutions, Zashch. Met., 1994, vol. 30, no. 6, pp. 620–623.
Pletnev, M.A., Shirobokov, I.B., Ovechkina, O.E., and Reshetnikov, S.M., Influence of tetraalkylammonium on the cathode hydrogen evolution in concentrated acid solutions, Zashch. Met., 1995, vol. 31, no. 4, pp. 351–355.
Nakanisi, K., Infrared Absorption Spectroscopy, Tokyo: Holden Day, 1962.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Hoboken, New Jersey: Wiley, 1986, 4th ed.
Dana, W. Mayo, Foil A. Miller, and Robert W. Hannah, Course Notes on the Interpretation of Infrared and Raman Spectra, New Jersey: Wiley, 2003.
Prosek, T., Nazarov, A., Thierry, U., and Serak, J., Corrosion mechanism of model zinc-magnesium alloys in atmospheric conditions, Corros. Sci., 2008, vol. 50, pp. 2216–2231.
Persson, D., Thierry, D., LeBozec, N., and Prosek, T., In situ infrared reflection spectroscopy studies of the initial atmospheric corrosion of Zn–Al–Mg coated steel, Corros. Sci., 2013, vol. 72, pp. 54–63.
Jayasree, R.S., Pillai, V.P., Mahadevan, NayarV.U., Odnevall, I., and Keresztury, G., Raman and infrared analysis of corrosion products on zinc NaZ-n4Cl(OH)6SO4 · 6H2O and Zn4Cl2(OH)4SO4 · 5H2O, Mater. Chem. Phys., 2006, vol. 99, pp. 474–478.
Diler, E., Rioual, S., Lescop, B., Thierry, D., and Rouvellou, B., Chemistry of corrosion products of Zn and MgZn pure phases under atmospheric conditions, Corros. Sci., 2012, vol. 65, pp. 178–186.
Hosseinpour, S. and Johnson, M., Vibrational spectroscopy in studies of atmospheric corrosion, Materials, 2017, vol. 10, p. 413.https://doi.org/10.3390/ma10040413
ACKNOWLEDGMENTS
This study was supported by the Russian Foundation for Basic Research, project r_a no. 16-42-180814.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors claim that they have no conflict of interest.
Additional information
Translated by N. Korovin
About this article
Cite this article
Kanunnikova, O.M., Aksenova, V.V., Pushkarev, B.E. et al. Peculiarities of Corrosion of a Zinc Coating in Neutral Media in the Presence of Inhibitors Based on Benzotriazole, Cyclohexylamine, and Morpholine. Russ. J. Non-ferrous Metals 60, 390–400 (2019). https://doi.org/10.3103/S1067821219040047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821219040047