Abstract
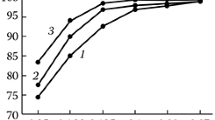
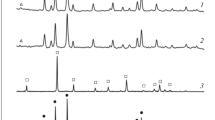
The synthesis of carnallite from the spent electrolyte of magnesium cells and a chlorine-magnesium solution is studied. The process parameters that ensure the formation of synthetic carnallite that meet the requirements of the magnesium industry are determined. Specifically, the synthesis temperature is 100–130°C, the initial concentration of magnesium chloride in a solution is 290–400 g/l, the consumption of spent electrolyte is 105% of the stoichiometric composition, and the particle size is 0.315 mm. The sequence of synthesis operations is as follows: heating of a chlorine-magnesium solution and a spent electrolyte to 110–120°C, their mixing in a reactor, electrolyte dissolution, the evaporation of a reaction mass during agitation, and drying.
Similar content being viewed by others
References
Lokshin, M.Z. and Makarov, G.S., Tsvetn. Met., 2006, no. 5, p. 46.
Strelets, Kh.L., Taits, A.Yu., and Gulyanitskii, B.S., Metallurgiya magniya (Metallurgy of Magnesium), Moscow: Metallurgizdat, 1960.
Shchelkonogov, A.A., Detkov, P.G., Mal’tsev N.A., et al., RF Patent 2182559, 2002.
Teterin, V.V., Freidlina, R.G., Gladikova, L.A., and Bezdolya, I.N., Zh. Prikl. Khim., 2007, no. 3, p. 521.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.Yu. Shirev, V.A. Lebedev, 2011, published in Izvestiya VUZ. Tsvetnaya Metallurgiya, 2011, No. 6, pp. 25–30.
About this article
Cite this article
Shirev, M.Y., Lebedev, V.A. New process of synthetic carnallite production. Russ. J. Non-ferrous Metals 52, 485–489 (2011). https://doi.org/10.3103/S1067821211060150
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821211060150