Abstract
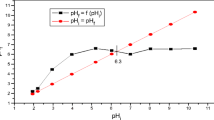
Characterization of coarse fractions of kaolin clay from two locations in Puolanka (Pihlajavaara and Poskimäki) was performed in order to find potential applications for these materials in water and wastewater treatment as low-cost adsorbents or as a raw material for other uses. The effects of wet and dry fractionation methods and the annealing of the sample on the properties of the coarse fractions were studied. The coarse fractions contained mainly quartz, while the kaolinite content was considered low. The Poskimäki (PM) clay had a higher specific surface area (SSA) and cation exchange capacities (CEC) than the Pihlajavaara (PV) clay due to a higher amount of iron. Annealing (800°C) decreased the SSA and CEC. The fractionation method had only a minor effect on particle size distribution. PV and PM colloidal suspensions had a negative zeta potential at natural pH values. Very small amounts of contained elements (Al, Si, Ca, Mg, K, Cd, Co, Fe, Mn, Cr, Ni, Cu, Zn, Pb, Ba) were dissolved from samples at natural pH values. PM clay could be utilized in water treatment for example as a raw material in iron oxide-coated sands. For this purpose, the wet fractionated samples had a higher content of iron than the dry fractionated samples.
Similar content being viewed by others
References
Gupta S.S., Bhattacharyya K.G., Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium, J. Environ. Manage., 2008, 87, 46–58
Nylund J., Sundberg A., Sundberg K., Dissolved and colloidal substances from mechamical pulp suspension — Interactions influencing the sterical stability, Colloids Surf., 2007, A301, 335–340
Bhattacharyya K.G., Gupta S.S., Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review, Adv. Colloid Interface Sci., 2008, 140, 114–131
Boujelben N., Bouzid J., Elouear Z., Feki M., et al., Phosphorus removal from aqueous solution using iron coated natural and engineered sorbents, J. Hazard. Mater., 2008, 151, 103–110
Boujelben N., Bouzid J., Elouear Z., Adsorption of nickel and copper onto natural iron-oxide coated sand from aqueous solutions: Study in single and binary systems, J. Hazard. Mater., 2009, 163, 376–382
Sabir B.B., Wild S., Bai J., Metakaolin and calcined clays as pozzolans for concrete: a review, Cem. Concr. Compos., 2001, 23, 441–454
Chapman H.D., Cation-exchange Capacity, In: Black C.A. (ed), Method of Soil Analysis, Part 2: Chemical and Microbiological Properties, Am. Soc. Agron., Madison, Wisconsin, 1965
Niskanen R., Jaakkola A., Estimation of cation exchange capacity in routine soil testing, J. Agric. Sci. Finl., 1986, 58, 1–7
Waychunas G.A., Kim C.S., Banfield J.F., Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms, J. Nanopart. Res., 2005, 7, 409–433
Suraj G., Iyer C.S.P., Lalithambika M., Adsorption of cadmium and copper by modified kaolinites, Appl. Clay Sci., 1998, 13, 293–306
Harland C.E., Ion exchange: Theory and Practice, 2nd ed., Royal Society of Chemistry Paperbacks, Cambridge, UK., 1994
Langmuir D., Aqueous Environmental Geohemistry, Prentice Hall, New Jersey, 1997
Buffle J., Complexation Reactions in Aquatic Systems, Ellis Horwood, Chichester, U.K., 1988
Shvarzman A., Kovler K., Schamban I., Grader, G., et al., Influence of chemical and phase composition of mineral admixtures on their pozzolanic acitivity, Adv. Cement Res., 2001, 13, 1–7
Hanna K., Adsorption of aromatic carboxylate compounds on the surface of synthesized iron oxide-coated sands, Appl. Geochem., 2007, 22, 2045–2053
Alkan M., Demirbas Ö., Dogan M., Electrokinetic properties of kaolinite in mono- and multivalent electrolyte solutions, Microporous Mesoporous Mater., 2005, 83, 51–59
Kosmulski M., The pH-dependent surface charging and points of zero charge V. Update, J. Colloid Interface Sci., 2011, 353, 1–15
Xu Y., Axe L., Synthesis and characterization of iron oxide-coated silica and its effect on metal adsorption, J. Colloid Interface Sci., 2005, 282, 11–19
Schroth B.K., Sposito G., Surface charge properties of kaolinite, Clays Clay Miner., 1997, 45: 85–91
Gregory J., Duan J., Hydrolyzing metal salts as coagulants, Pure Appl. Chem., 2001, 73, 2017–2026
Gupta V.K., Saini V.K., Jain N., Adsorption of As (III) from aqueous solutions by iron oxide-coated sand, J. Colloid Interface Sci., 2005, 288, 55–60
Guo H., Stüben D., Berner Z., Arsenic removal from water using natural iron mineral-quartz sand columns, Sci. Total Environ., 2007, 377, 142–151
Ding C., Yang X., Liu W., Chang Y., et al., Removal of natural organic matter using surfactant-modified iron oxide-coated sand, J. Hazard. Mater., 2010, 174, 567–572
Song S., Lopez-Valdivieso A., Hernandez-Campos D.J., Peng C., et al., Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite, Water Res., 2006, 40, 364–372
Özacar M., Sengil I.A., Enhancing phosphate removal from wastewater by using polyelectrolytes and clay injection, J. Hazard. Mater., 2003, 100, 131–146
Shen Y., Treatment of low-turbidity water by sweep coagulation using clay, Sep. Sci. Technol., 2002, 37, 2739–2744
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Leiviskä, T., Gehör, S., Eijärvi, E. et al. Characteristics and potential applications of coarse clay fractions from Puolanka, Finland. cent.eur.j.eng 2, 239–247 (2012). https://doi.org/10.2478/s13531-011-0067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s13531-011-0067-9