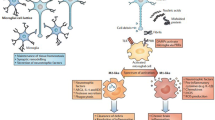
Abstract
Osteopontin (OPN) is a pro-inflammatory cytokine that can be secreted from many cells including activated macrophages and T-lymphocytes. Elevated levels of osteopontin in the plasma, cerebrospinal fluid or brain of individuals with neurodegenerative diseases such as multiple sclerosis (MS), Parkinson’s and Alzheimer’s disease and more recently in HIV-associated neurocognitive disorder has been reported. However, except for the case of MS, little is known regarding the molecular mechanisms by which OPN may exacerbate disease. Alternatively, OPN through its ability to promote cell survival may in some contexts function in the brain in a protective capacity. OPN has several protein motifs that allow it to engage with several different signaling pathways involved in immunity and inflammation. A better understanding of the cellular pathways that are regulated by OPN in cells of the central nervous system is required to uncover its putative role in neuronal homeostasis.
Similar content being viewed by others
References
Jansson M., Panoutsakopoulou V., Baker J., Klein L., Cantor H., Cutting edge: Attenuated experimental autoimmune enchephalomyelitis in eta-1/osteopontin-deficient mice, J. Immunol., 2002, 168, 2096–2099
Hur E. M., Youssef S., Haws M. E., Zhang S. Y., Sobel R. A., Steinman L., Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells, Nat. Immunol., 2006, 8, 74–83
Wung J. K., Perry G., Kowalski A., Harris P. L., Bishop G. M., Trivedi M. A., et al., Increased expression of the remodeling- and tumorigenic-associated factor osteopontin in pyramidal neurons of the Alzheimer’s disease brain, Curr. Alzheimer Res., 2007, 4, 67–72
Wirths O., Breyhan H., Marcello A., Cotel M. C., Brück W., Bayer T. A., Inflammatory changes are tightly associated with neurodegeneration in the brain and spinal cord of the APP/PS1KI mouse model of Alzheimer’s disease, Neurobiol. Aging, 2010, 31, 747–757
Comi C., Carecchio M., Chiocchetti A., Nicola S., Galimberti D., Fenoglio C., et al., Osteopontin is increased in the cerebrospinal fluid of patients with Alzheimer’s disease and its levels correlate with cognitive decline, J. Alzheimers Dis., 2010, 19, 1143–1148
Maetzler W., Berg D., Schalamberidze N., Melms A., Schott K., Mueller J. C., et al., Osteopontin is elevated in Parkinson’s disease and its absence leads to reduced neurodegeneration in the MPTP model, Neurobiol. Dis., 2007, 25, 473–482
Iczkiewicz J., Jackson M. J., Smith L. A., Rose S., Jenner P., Osteopontin expression in substantia nigra in MPTP-treated primates and in Parkinson’s disease, Brain Res., 2006, 1118, 239–250
Mattson N., Rüetschi U, Pijnenburg Y. A., Blankenstein M. A., Podust V. N., Li S., et al., Novel cerebrospinal fluid biomarkers of axonal degeneration in frontotemporal dementia, Mol. Med. Report, 2008, 1, 757–761
Brown A., Islam T., Adams R., Nerle S., Kamara M., Eger C., et al., Osteopontin enhances HIV replication and is increased in the brain and cerebrospinal fluid of HIV-infected individuals, J. Neurovirol., 2011, 17, 382–392
McArthur J. C., Steiner J., Sacktor N., Nath A., Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap, Ann. Neurol., 2010, 67, 699–714
Navia B. A., Jordan B. D., Price R. W., The AIDS dementia complex: I. Clinical features, Ann. Neurol., 1986, 19, 517–524
Heaton R. K., Franklin D. R., Ellis R. J., McCutchan J. A., Letendre S. L., et al., HIV-associated neurocognitive disorder before and during the era of combination antiretroviral therapy: differences in rates, nature and predictors, J. Neurovirol., 2011, 17, 3–16
Heaton R. K., Clifford D. B., Franklin D. R. Jr., Woods S. P., Ake C., Vaida F., et al., HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study, Neurology, 2010, 75, 2087–2096
Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., et al., Updated research nosology for HIV-associated neurocognitive disorders, Neurology, 2007, 69, 1789–1799
Kraft-Terry S. D., Stothert A. R., Buch S., Gendelman H. E., HIV-1 neuroimmunity in the era of antiretroviral therapy, Neurobiol. Dis., 2010, 37, 542–548
Burdo T. H., Wood M. R., Fox H. S., Osteopontin prevents monocyte recirculation and apoptosis, J. Leukoc. Biol., 2007, 81, 1504–1511
Marcondes M. C., Lanigan C. M., Burdo T. H., Watry D. D., Fox H. S., Increased expression of monocyte CD44v6 correlates with the development of encephalitis in rhesus macaques infected with simian immunodeficiency virus, J. Infect. Dis., 2008, 197, 1567–1576
Burdo T. H., Ellis R. J., Fox H. S., Osteopontin is increased in HIV-associated dementia, J. Infect. Dis., 2008, 198, 715–722
Brown A., Gartner S., Kawano T., Benoit N., Cheng-Mayer C., HLA-A2 down-regulation on primary human macrophages infected with an M-tropic EGFP-tagged HIV-1 reporter virus, J. Leukoc. Biol., 2005, 78, 675–685
Brown A., Zhang H., Lopez P., Pardo C. A., Gartner S., In vitro modeling of the HIV-macrophage reservoir, J. Leukoc. Biol., 2006, 80, 1127–1135
Ziegler-Heitbrock H. W. L., Fingerle G., Ströbel M., Schraut W., Stelter F., Schütt C., et al., The novel subset of CD14+ CD16+ blood monocytes exhibits features of tissue macrophages, Eur. J. Immunol., 1993, 23, 2053–2058
Marder K., Albert S. M., McDermott M. P., McArthur J. C., Schifitto G., Selnes O. A., et al., Inter-rater reliability of a clinical staging of HIV-associated cognitive impairment, Neurology, 2003, 60, 1467–1473
Sacktor N., McDermott M. P., Marder K., Schifitto G., Selnes O. A., McArthur J. C., et al., HIV-associated cognitive impairment before and after the advent of combination therapy, J. Neurovirol., 2002, 8, 136–142
Anborgh P. H., Wilson S. M., Tuck A. B., Winquist E., Schmidt N., Hart R., et al., New dual monoclonal ELISA for measuring plasma osteopontin as a biomarker associated with survival in prostate cancer: clinical validation and comparison of multiple ELISAs, Clin. Chem., 2009, 55, 895–903
Letendre S., Marquie-Beck J., Capparelli E., Best B., Clifford D., Collier A. C., et al., Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system, Arch. Neurol., 2008, 65, 65–70
Lampe M. A., Patarca R., Iregui M. V., Cantor H., Polyclonal B cell ativation by the Eta-1 cytokine and the development of systemic autoimmune disease, J. Immunol., 1991, 147, 2902–2906
Saito Y., Kon S., Fujiwara Y., Nakayama Y., Kurotaki D., Fukuda N., et al., Osteopontin small interfering RNA protects mice from fulminant hepatitis, Hum. Gene Ther., 2007, 18, 1205–1214
Rollo E. E., Hempson S. J., Bansal A., Tsao E., Habib J., Rittling S. R., et al., The cytokine osteopontin modulates the severity of rotavirus diarrhea, J. Virol., 2005, 79, 3509–3516
Patarca R., Saavedra R. A., Cantor H., Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene, Crit. Rev. Immunol., 1993, 13, 225–246
Nau G. J., Liaw L., Chupp G. L., Berman J. S., Hogan B. L., Young R. A., Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin, Infect. Immun., 1999, 67, 4223–4230
Weber G. F., Ashkar S., Glimcher M. J., Cantor H., Receptor-ligand interaction between CD44 and osteopontin/Eta-1, Science, 1996, 271, 509–512
Patarca R., Freeman G. J., Singh R. P., Wei F. Y., Durfee T., Blattner F., et al., Structural and functional studies of the early T-lymphocyte activation 1 (Eta-1) gene, J. Exp. Med., 1989, 170, 145–161
Ashkar S., Weber G. F., Panoutsakopoulou V., Sanchirico M. E., Jannson M., Zawaideh S., et al., Eta-1 (Osteopontin): An early component of type-1 (cell-mediated) immunity, Science, 2000, 287, 860–864
Wang K. X., Denhardt D. T., Osteopontin: Role in immune regulation and stress responses, Cytokine Growth Factor Rev., 2008, 19, 333–345
Shinohara M. L., Lu L., Bu J., Werneck M. B., Kobayashi K. S., Glimcher L. H., et al., Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells, Nat. Immunol., 2006, 7, 498–506
Morimoto J., Sato K., Nakayama Y., Kimura C., Kajino K., Matsui Y., et al., Osteopontin modulates the generation of memory CD8+ T cells during influenza virus infection, J. Immunol., 2011, 187, 5671–5683
Chabas D., Baranzini S. E., Mitchell D., Bernard C. C., Rittling S. R., Denhardt D. T., et al., The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease, Science, 2001, 294, 1731–1735
Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., et al., Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis, Nat. Med., 2002, 8, 500–508
Vogt M. H., Floris S., Killestein J., Knol D. L., Smits M., Barkhof F., et al., Osteopontin levels and increased disease activity in relapsing remitting multiple sclerosis patients, J. Neuroimmunol., 2004, 155, 155–160
Comabella M., Pericot I., Goertsches R., Nos C., Castillo M., Blas Navarro J., et al., Plasma osteopontin levels in multiple sclerosis, J. Neuroimmunol., 2005, 158, 231–239
Braitch M., Nunan R., Niepel G., Edwards L. J., Constantinescu C. S., Increased osteopontin levels in the cerebrospinal fluid of patients with multiple sclerosis, Arch. Neurol., 2008, 65, 633–635
Tiberti N., Hainard A., Lejon V., Robin X., Ngoyi D. M., Turck N., et al., Discovery and verification of osteopontin and Beta-2-microglobulin as promising markers for staging human African trypanosomiasis, Mol. Cell. Proteomics, 2010, 9, 2783–2795
Sodek J., Ganss B., McKeee M. D., Osteopontin, Crit. Rev. Oral Biol. Med., 2000, 11, 279–303
Denhardt, D. T., Guo X, Osteopontin: A protein with diverse functions, FASEB J., 1993, 7, 1475–1482
Bellahcène A., Castronovo V., Ogbureke K. U., Fisher L. W., Fedarko N. S., Small integrin-binding ligand N-liked glycoproteins (SIBLINGs): multifunctional proteins in cancer, Nat. Rev. Cancer, 2008, 8, 212–226
Kazanecki C. C., Uzwiak D. J., Denhardt D. T., Control of osteopontin signaling and function by post-translational phosphorylation and protein folding, J. Cell. Biochem., 2007, 102, 912–924
Ransohoff R. M., Cardona A. E., The myeloid cells of the central nervous system parenchyma, Nature, 2010, 468, 253–262
Suzuki K., Takeyama S., Sakai Y., Yamada S., Shinoda H., Current topics in pharmacological research on bone metabolism: inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts, J. Pharmacol. Sci., 2006, 100, 189–194
Ofotokun I., Weitzmann M. N., HIV and bone metabolism, Discovery Med., 2011, 11, 385–393
Ofotokun I., Weitzmann M. N., HIV: inflammation and bone, Curr. HIV/AIDS Rep., 2012, 9, 16–25
Clowes J., Riggs B. L., Khosia S., The role of immune system in the pathophysiology of osteoporosis, Immunol. Rev., 2005, 208, 207–227
Pirraco R. P., Reis R. L., Marques A. P., Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs, J. Tissue Eng. Regen. Med., 2012, doi: 10.1002/term.535 [Epub ahead of print]
Brown T. T., Ross A. C., Storer N., Labbato D., McComsey G. A., Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens, Antivir. Ther., 2011, 16, 1063–1072
Vikulina T., Fan X., Yamaguchi M., Roser-Page S., Zayzafoon M., Guidot D. M., et al., Alterations in the immuno-skeletal interface drive bone distruction in HIV-1 transgenic rats, Proc. Natl. Acad. Sci. USA, 2010, 107, 13848–13853
Haskelberg H., Carr A., Emery S., Bone turnover markers in HIV disease, AIDS Rev., 2011, 13, 240–250
Walker Harris B., Brown T. T., Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies, J. Infect. Dis., 2012, 205(Suppl. 3), S391–398
Coiras M., López-Huertas M. R., Sánchez del Cojo M., Mateos E., Alcamí J., Dual role of host cell factors in HIV-1 replication: restriction and enhancement of the viral cycle, AIDS Rev., 2010, 12, 103–112
Shin S. L., Cha J. H., Chun M. H., Chung J. W., Lee M. Y., Expression of osteopontin mRNA in the adult rat brain, Neurosci. Lett., 1999, 273, 73–76
Ichikawa H., Itota T., Nishitani Y., Torii Y., Inoue K., Sugimoto T., Osteopontin-immunoreactive primary sensory neurons in the rat spinal and trigeminal nervous systems, Brain Res., 2000, 863, 276–281
Glezer I., Bittencourt J. C., Rivest S., Neuronal expression of Cd36, Cd44, and Cd83 antigen transcripts maps to distinct and specific murine brain circuits, J. Comp. Neurol., 2009, 517, 906–924
Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T., 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues, Cell Tissue Res., 1988, 251, 23–30
van Velthoven C. T., Heijnen C. J., van Bel F., Kavelaars A., Osteopontin enhances endogenous repair after neonatal hypoxic-ischemic brain injury, Stroke, 2011, 42, 2294–2301
Morinobu M., Ishijima M., Rittling S. R., Tsuji K., Yamamoto H., Nifuji A., et al., Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo, J. Bone Miner. Res., 2003, 18, 1706–1715
McKee M. D., Addison W. N., Kaartinen M. T., Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton, Cells Tissues Organs, 2005, 181, 176–188
Iseki S., Wilkie A. O., Heath J. K., Ishimaru T., Eto K., Morriss-Kay G. M., Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2, Development, 1997, 124, 3375–3384
Hazenberg M. D., Stuart J. W., Otto S. A., Borleffs J. C., Boucher C. A., de Boer R. J., et al., T-cell division in human immunodeficiency virus (HIV-1) infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART), Blood, 2000, 95, 249–255
Connoly N., Riffler S., Rinaldo C., Proinflammatory cytokines in HIV disease — a review and rationale for new therapeutic approaches, AIDS Rev., 2005, 7, 168–180
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Brown, A. Osteopontin: A key link between immunity, inflammation and the central nervous system. Translat.Neurosci. 3, 288–293 (2012). https://doi.org/10.2478/s13380-012-0028-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s13380-012-0028-7