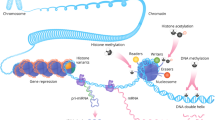
Abstract
Accumulating evidence from the field of neuroscience indicates a crucial role for epigenetic regulation of gene expression in development and aging of nervous system and suggests that aberrations in the epigenetic machinery are involved in the etiology of psychiatric disorders. Epidemiologic evidence on epigenetics in psychiatry, however, is currently very sparsely available, but is consistent with a mediating role for epigenetic mechanisms in bringing together inherited and acquired risk factors into a neurodevelopmental etiological model of psychiatric disorders. Here, we review evidence from the epidemiological and neuroscience literature, and aim to converge the evidence into an etiological model of psychiatric disorders that encompasses environmental, genetic and epigenetic contributions. Given the dynamic nature of the epigenetic machinery and the potential reversibility of epigenetic modifications, future well-designed interdisciplinary and translational studies will be of key importance in order to identify new targets for prevention and therapeutic strategies.
Similar content being viewed by others
References
Miller, G., Epigenetics. The seductive allure of behavioral epigenetics. Science, 2010. 329(5987): p. 24–27.
Petronis, A., Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature, 2010. 465(7299): p. 721–727.
Jaenisch, R. and A. Bird, Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet, 2003. 33Suppl: p. 245–254.
Berger, S.L., The complex language of chromatin regulation during transcription. Nature, 2007. 447(7143): p. 407–412.
Miller, B.H. and C. Wahlestedt, MicroRNA dysregulation in psychiatric disease. Brain Res, 2010. 1338: p. 89–99.
Feinberg, A.P., Phenotypic plasticity and the epigenetics of human disease. Nature, 2007. 447(7143): p. 433–440.
Okano, M., et al., DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 1999. 99(3): p. 247–257.
Li, E., T.H. Bestor, and R. Jaenisch, Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell, 1992. 69(6): p. 915–926.
Weaver, J.R., M. Susiarjo, and M.S. Bartolomei, Imprinting and epigenetic changes in the early embryo. Mamm Genome, 2009. 20(9–10): p. 532–543.
Hsieh, J. and A.J. Eisch, Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiology of disease, 2010. 39(1): p. 73–84.
Yu, Y., P. Casaccia, and Q.R. Lu, Shaping the oligodendrocyte identity by epigenetic control. Epigenetics, 2010. 5(2): p. 124–128.
Hsieh, J. and F.H. Gage, Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol, 2005. 17(6): p. 664–671.
Levenson, J.M. and J.D. Sweatt, Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci, 2006. 63(9): p. 1009–1016.
Renthal, W. and E.J. Nestler, Epigenetic mechanisms in drug addiction. Trends Mol Med, 2008. 14(8): p. 341–350.
Day, J.J. and J.D. Sweatt, DNA methylation and memory formation. Nat Neurosci, 2010. 13(11): p. 1319–1323.
Feng, J., et al., Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci, 2010. 13(4): p. 423–430.
Flavell, S.W. and M.E. Greenberg, Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci, 2008. 31: p. 563–590.
MacDonald, J.L. and A.J. Roskams, Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol, 2009. 88(3): p. 170–183.
Bale, T.L., et al., Early life programming and neurodevelopmental disorders. Biol Psychiatry, 2010. 68(4): p. 314–319.
Fraga, M.F. and M. Esteller, Epigenetics and aging: the targets and the marks. Trends Genet, 2007. 23(8): p. 413–418.
Chouliaras, L., et al., Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Progress in neurobiology, 2010. 90(4): p. 498–510.
Christensen, B.C., et al., Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet, 2009. 5(8): p. e1000602.
Jodo, E., C. Chiang, and G. Aston-Jones, Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience, 1998. 83(1): p. 63–79.
Chouliaras, L., et al., Caloric restriction attenuates age-related changes of DNA methyltransferase 3a in mouse hippocampus. Brain, behavior, and immunity, 2010.
Colman, R.J., et al., Caloric restriction delays disease onset and mortality in rhesus monkeys. Science, 2009. 325(5937): p. 201–204.
Mattson, M.P., et al., Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol Aging, 2002. 23(5): p. 695–705.
Sohal, R.S. and R. Weindruch, Oxidative stress, caloric restriction, and aging. Science, 1996. 273(5271): p. 59–63.
Rutten, B.P., et al., Caloric restriction and aging but not overexpression of SOD1 affect hippocampal volumes in mice. Mechanisms of ageing and development, 2010. 131(9): p. 574–579.
Chouliaras, L., et al., Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiology of aging, 2011.
Liu, L., et al., DNA methylation impacts on learning and memory in aging. Neurobiol Aging, 2009. 30(4): p. 549–560.
Peleg, S., et al., Altered histone acetylation is associated with agedependent memory impairment in mice. Science, 2010. 328(5979): p. 753–756.
Longo, V.D. and B.K. Kennedy, Sirtuins in aging and age-related disease. Cell, 2006. 126(2): p. 257–268.
Mehler, M.F., Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Progress in neurobiology, 2008. 86(4): p. 305–341.
Khan, N.L. and N.W. Wood, Prader-Willi and Angelman syndromes: update on genetic mechanisms and diagnostic complexities. Curr Opin Neurol, 1999. 12(2): p. 149–154.
Lalande, M. and M.A. Calciano, Molecular epigenetics of Angelman syndrome. Cell Mol Life Sci, 2007. 64(7–8): p. 947–960.
Christodoulou, J. and L.S. Weaving, MECP2 and beyond: phenotypegenotype correlations in Rett syndrome. J Child Neurol, 2003. 18(10): p. 669–674.
Dimitropoulos, A. and R.T. Schultz, Autistic-like symptomatology in Prader-Willi syndrome: a review of recent findings. Curr Psychiatry Rep, 2007. 9(2): p. 159–164.
van Os, J., B.P. Rutten, and R. Poulton, Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull, 2008. 34(6): p. 1066–1082.
Petronis, A., et al., Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance? Schizophr Bull, 2003. 29(1): p. 169–178.
Suzuki, K., [Japanese adolescents’ drinking and problem drinking]. Nippon Rinsho, 1997. 55Suppl: p. 522–526.
McClellan, J., et al., Clinical characteristics related to severity of sexual abuse: a study of seriously mentally ill youth. Child Abuse Negl, 1995. 19(10): p. 1245–1254.
Sansone, R.A., L.A. Sansone, and E.L. Righter, Panic disorder: the ultimate anxiety. J Womens Health, 1998. 7(8): p. 983–989.
Mill, J., et al., Evidence for monozygotic twin (MZ) discordance in methylation level at two CpG sites in the promoter region of the catechol-O-methyltransferase (COMT) gene. Am J Med Genet B Neuropsychiatr Genet, 2006. 141(4): p. 421–425.
Lepine, J.P. and S. Bouchez, Epidemiology of depression in the elderly. Int Clin Psychopharmacol, 1998. 13Suppl 5: p. S7–12.
Barbieri, N.B., Psychoanalytic contributions to the study of gender issues. Can J Psychiatry, 1999. 44(1): p. 72–76.
Dempster, E.L., et al., Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Human molecular genetics, 2011. 20(24): p. 4786–4796.
Saito, T., [Psychiatric studies on alcoholism in Japan]. Arukoru Kenkyuto Yakubutsu Ison, 1995. 30(6): p. 411–425.
Rutten, B.P. and J. Mill, Epigenetic mediation of environmental influences in major psychotic disorders. Schizophrenia bulletin, 2009. 35(6): p. 1045–1056.
Jirtle, R.L. and M.K. Skinner, Environmental epigenomics and disease susceptibility. Nature reviews. Genetics, 2007. 8(4): p. 253–262.
Fraga, M.F., et al., Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A, 2005. 102(30): p. 10604–10609.
Sherazi, R., et al., What’s new? The clinical epidemiology of bipolar I disorder. Harv Rev Psychiatry, 2006. 14(6): p. 273–284.
Bijl, R.V., et al., Gender and age-specific first incidence of DSM-III-R psychiatric disorders in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Soc Psychiatry Psychiatr Epidemiol, 2002. 37(8): p. 372–379.
Bijl, R.V., A. Ravelli, and G. van-Zessen, Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Soc-Psychiatry-Psychiatr-Epidemiol, 1998. 33(12): p. 587–595.
Wittchen, H.U., M.B. Stein, and R.C. Kessler, Social fears and social phobia in a community sample of adolescents and young adults: prevalence, risk factors and co-morbidity. Psychol Med, 1999. 29(2): p. 309–323.
Polanczyk, G. and L.A. Rohde, Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry, 2007. 20(4): p. 386–392.
Fombonne, E., Epidemiology of pervasive developmental disorders. Pediatr Res, 2009. 65(6): p. 591–598.
Fombonne, E., Epidemiological trends in rates of autism. Mol Psychiatry, 2002. 7Suppl 2: p. S4–S6.
Nelson, C.B. and H.U. Wittchen, DSM-IV alcohol disorders in a general population sample of adolescents and young adults. Addiction, 1998. 93(7): p. 1065–1077.
van Os, J. and S. Kapur, Schizophrenia. Lancet, 2009. 374(9690): p. 635–645.
van Os, J., G. Kenis, and B.P. Rutten, The environment and schizophrenia. Nature, 2010. 468(7321): p. 203–212.
McCarthy, M.M., et al., The epigenetics of sex differences in the brain. The Journal of neuroscience: the official journal of the Society for Neuroscience, 2009. 29(41): p. 12815–12823.
Schwarz, J.M., B.M. Nugent, and M.M. McCarthy, Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 151(10): p. 4871–4881.
Waggoner, D., Mechanisms of disease: epigenesis. Semin Pediatr Neurol, 2007. 14(1): p. 7–14.
Monteiro, J., et al., Commitment to X inactivation precedes the twinning event in monochorionic MZ twins. Am J Hum Genet, 1998. 63(2): p. 339–346.
Manning, N., The influence of twinning on cardiac development. Early Hum Dev, 2008. 84(3): p. 173–179.
Hall, L.L. and J.B. Lawrence, The cell biology of a novel chromosomal RNA: chromosome painting by XIST/Xist RNA initiates a remodeling cascade. Semin Cell Dev Biol, 2003. 14(6): p. 369–378.
Loat, C.S., et al., X inactivation as a source of behavioural differences in monozygotic female twins. Twin Res, 2004. 7(1): p. 54–61.
Peerbooms, O.L., et al., No major role for X-inactivation in variations of intelligence and behavioral problems at middle childhood. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics, 2010. 153B(7): p. 1311–1317.
Rosa, A., et al., Differential methylation of the X-chromosome is a possible source of discordance for bipolar disorder female monozygotic twins. Am J Med Genet B Neuropsychiatr Genet, 2008. 147B(4): p. 459–462.
Franklin, T.B., et al., Epigenetic transmission of the impact of early stress across generations. Biological psychiatry, 2010. 68(5): p. 408–415.
Malaspina, D., et al., Growth and schizophrenia: aetiology, epidemiology and epigenetics. Novartis Found Symp, 2008. 289: p. 196–203; discussion 203–7, 238–40.
Delahanty, R.J., et al., Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol Psychiatry, 2009.
Anney, R.J., et al., Parent of origin effects in attention/deficit hyperactivity disorder (ADHD): analysis of data from the international multicenter ADHD genetics (IMAGE) program. Am J Med Genet B Neuropsychiatr Genet, 2008. 147B(8): p. 1495–1500.
De Luca, V., et al., Parent of origin effect and differential allelic expression of BDNF Val66Met in suicidal behaviour. World J Biol Psychiatry.
De Luca, V., et al., Differential expression and parent-of-origin effect of the 5-HT2A receptor gene C102T polymorphism: analysis of suicidality in schizophrenia and bipolar disorder. Am J Med Genet B Neuropsychiatr Genet, 2007. 144B(3): p. 370–374.
Goos, L.M., P. Ezzatian, and R. Schachar, Parent-of-origin effects in attention-deficit hyperactivity disorder. Psychiatry Res, 2007. 149(1–3): p. 1–9.
Bassett, S.S., et al., Further evidence of a maternal parent-of-origin effect on chromosome 10 in late-onset Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet, 2006. 141B(5): p. 537–540.
Kosztolanyi, G., [First decade of post-genomic era. Hopes, disappointments, new answers]. Orvosi hetilap, 2010. 151(51): p. 2099–2104.
Grether, J.K., et al., Risk of autism and increasing maternal and paternal age in a large north American population. Am J Epidemiol, 2009. 170(9): p. 1118–1126.
Reichenberg, A., et al., Advancing paternal and maternal age are both important for autism risk. Am J Public Health, 2010. 100(5): p. 772–773; author reply 773.
Croen, L.A., et al., Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med, 2007. 161(4): p. 334–340.
Reichenberg, A., et al., Advancing paternal age and autism. Arch Gen Psychiatry, 2006. 63(9): p. 1026–1032.
Miller, B., et al., Meta-analysis of Paternal Age and Schizophrenia Risk in Male Versus Female Offspring. Schizophr Bull, 2010.
Saha, S., et al., Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med, 2009. 6(3): p. e40.
Saha, S., et al., Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr Res, 2009. 115(2–3): p. 130–135.
Wichers, M., et al., Mechanisms of gene-environment interactions in depression: evidence that genes potentiate multiple sources of adversity. Psychological medicine, 2009. 39(7): p. 1077–1086.
Wichers, M.C., et al., Prenatal life and post-natal psychopathology: evidence for negative gene-birth weight interaction. Psychol Med, 2002. 32(7): p. 1165–1174.
Oh, G. and A. Petronis, Environmental studies of schizophrenia through the prism of epigenetics. Schizophr Bull, 2008. 34(6): p. 1122–1129.
Crow, T.J., How and why genetic linkage has not solved the problem of psychosis: review and hypothesis. Am J Psychiatry, 2007. 164(1): p. 13–21.
Wong, A.H., Gottesman, II, and A. Petronis, Phenotypic differences in genetically identical organisms: the epigenetic perspective. Human molecular genetics, 2005. 14Spec No 1: p. R11–R18.
Khashan, A.S., et al., Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry, 2008. 65(2): p. 146–152.
Huttunen, M.O. and P. Niskanen, Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry, 1978. 35(4): p. 429–431.
Van Os, J. and J.-P. Selten, Prenatal exposure to maternal stress and subsequent schizophrenia: The May 1940 invasion of The Netherlands. British Journal of Psychiatry, 1998. 172: p. 324–326.
Susser, E., et al., Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry, 1996. 53(1): p. 25–31.
Xu, M.Q., et al., Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull, 2009. 35(3): p. 568–576.
Brown, A.S., et al., Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Archives of General Psychiatry, 2007. 64(1): p. 31–39.
Hollister, J.M., P. Laing, and S.A. Mednick, Rhesus incompatibility as a risk factor for schizophrenia in male adults. Arch Gen Psychiatry, 1996. 53(1): p. 19–24.
McGrath, J.J., et al., Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study Archives of General Psychiatry, in press.
Mortensen, P.B., et al., Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biological Psychiatry, 2007. 61(5): p. 688–693.
Brown, A.S., et al., Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. American Journal of Psychiatry, 2005. 162(4): p. 767–773.
Brown, A.S. and E.J. Derkits, Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry, 2010. 167(3): p. 261–280.
Sorensen, H.J., et al., Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophrenia Bulletin, 2009. 35(3): p. 631–637.
Clarke, M.C., et al., Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. American Journal of Psychiatry, 2009. 166(9): p. 1025–1030.
Sorensen, H.J., et al., Do hypertension and diuretic treatment in pregnancy increase the risk of schizophrenia in offspring? Am J Psychiatry, 2003. 160(3): p. 464–468.
Sorensen, H.J., et al., Association between prenatal exposure to analgesics and risk of schizophrenia. Br J Psychiatry, 2004. 185: p. 366–371.
Damm, K., ErbA: tumor suppressor turned oncogene? FASEB J, 1993. 7(10): p. 904–909.
Meltzer, H.Y., The mechanism of action of novel antipsychotic drugs. Schizophr Bull, 1991. 17(2): p. 263–287.
Smits, L., et al., Association between short birth intervals and schizophrenia in the offspring. Schizophr Res, 2004. 70(1): p. 49–56.
Ohlund, L.S. and C.M. Hultman, Early parental death: relation to electrodermal orienting response and gender in schizophrenia. Schizophr Res, 1992. 7(2): p. 125–133.
Selten, J.P., et al., Schizophrenia and 1957 pandemic of influenza: meta-analysis. Schizophrenia Bulletin, 2010. 36(2): p. 219–228.
Selten, J.P., et al., No relationship between risk of schizophrenia and prenatal exposure to stress during the Six-Day War or Yom Kippur War in Israel. Schizophrenia Research, 2003. 63(1–2): p. 131–135.
McGrath, J., et al., Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophrenia Research, 2003. 63(1–2): p. 73–78.
Cannon, T.D., et al., A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol, 1999. 11(3): p. 467–485.
Buka, S.L., et al., Maternal infections and subsequent psychosis among offspring. Archives of General Psychiatry, 2001. 58(11): p. 1032–1037.
Brown, A.S., et al., No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? American Journal of Psychiatry, 2006. 163(12): p. 2178–2180.
Heijmans, B.T., et al., Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A, 2008. 105(44): p. 17046–17049.
Butler, P.D., et al., Prenatal nutritional deprivation as a risk factor in schizophrenia: preclinical evidence. Neuropsychopharmacology, 1994. 11(4): p. 227–235.
Tienari, P., et al., The Finnish adoptive family study of schizophrenia. Implications for family research. Br J Psychiatry Suppl, 1994(23): p. 20–26.
Wahlberg, K.E., et al., Gene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry, 1997. 154(3): p. 355–362.
Tienari, P., et al., Genotype-environment interaction in schizophrenia-spectrum disorder. Long-term follow-up study of Finnish adoptees. British Journal of Psychiatry, 2004. 184: p. 216–222.
Tienari, P., et al., Interaction of genetic and psychosocial factors in schizophrenia. Acta Psychiatr Scand Suppl, 1985. 319: p. 19–30.
Carter, J.W., et al., MMPI variables predictive of schizophrenia in the Copenhagen High-Risk Project: a 25-year follow-up. Acta Psychiatr Scand, 1999. 99(6): p. 432–440.
Schreier, A., et al., Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry, 2009. 66(5): p. 527–536.
Kessler, R.C., et al., Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry, 2010. 197: p. 378–385.
McLaughlin, K.A., et al., Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Arch Gen Psychiatry, 2010. 67(2): p. 124–132.
Green, J.G., et al., Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry, 2010. 67(2): p. 113–123.
Bruffaerts, R., et al., Childhood adversities as risk factors for onset and persistence of suicidal behaviour. Br J Psychiatry, 2010. 197(1): p. 20–27.
Weaver, I.C., et al., Epigenetic programming by maternal behavior. Nature neuroscience, 2004. 7(8): p. 847–854.
Weaver, I.C., et al., Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci, 2005. 25(47): p. 11045–11054.
Weaver, I.C., M.J. Meaney, and M. Szyf, Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A, 2006. 103(9): p. 3480–3485.
McGowan, P.O., et al., Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience, 2009. 12(3): p. 342–348.
Arseneault, L., et al., Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry, 2004. 184: p. 110–117.
Henquet, C., et al., Gene-environment interplay between cannabis and psychosis. Schizophrenia bulletin, 2008. 34(6): p. 1111–1121.
Murray, R.M., et al., Cannabis, the mind and society: the hash realities. Nature reviews. Neuroscience, 2007. 8(11): p. 885–895.
Houston, J.E., et al., Childhood sexual abuse, early cannabis use, and psychosis: testing an interaction model based on the National Comorbidity Survey. Schizophr Bull, 2008. 34(3): p. 580–585.
Caspi, A., et al., Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry, 2005. 57(10): p. 1117–1127.
Henquet, C., et al., COMT ValMet moderation of cannabisinduced psychosis: a momentary assessment study of ’switching on’ hallucinations in the flow of daily life. Acta psychiatrica Scandinavica, 2009. 119(2): p. 156–160.
van Winkel, R., Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Archives of general psychiatry, 2011. 68(2): p. 148–157.
De Hert, M., et al., Effects of cannabis use on age at onset in schizophrenia and bipolar disorder. Schizophr Res.
G.R.O.U.P., Evidence that Familial Liability for Psychosis is Expressed as Differential Sensitivity to Cannabis: an Analysis of Patient-Sibling and Sibling-Control Pairs. Archives of General Psychiatry, 2010. in press.
Chevaleyre, V., K.A. Takahashi, and P.E. Castillo, Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Annu Rev Neurosci, 2006.
Villares, J., Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience, 2007. 145(1): p. 323–334.
Ellgren, M., S.M. Spano, and Y.L. Hurd, Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology, 2007. 32(3): p. 607–615.
Casu, M.A., et al., Effect of delta9-tetrahydrocannabinol on phosphorylated CREB in rat cerebellum: an immunohistochemical study. Brain Res, 2005. 1048(1–2): p. 41–47.
Fernandez-Ruiz, J., et al., Cannabinoids and gene expression during brain development. Neurotox Res, 2004. 6(5): p. 389–401.
Mato, S., et al., A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci, 2004. 7(6): p. 585–586.
Scallet, A.C., Neurotoxicology of cannabis and THC: a review of chronic exposure studies in animals. Pharmacol Biochem Behav, 1991. 40(3): p. 671–676.
Heath, R.G., et al., Cannabis sativa: effects on brain function and ultrastructure in rhesus monkeys. Biol Psychiatry, 1980. 15(5): p. 657–690.
Hoffman, A.F., et al., Functional tolerance and blockade of longterm depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci, 2003. 23(12): p. 4815–4820.
Featherstone, R.E., S. Kapur, and P.J. Fletcher, The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry, 2007. 31(8): p. 1556–1571.
Uslaner, J., et al., Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. European Journal of Neuroscience, 2001. 13(10): p. 1977–1983.
Bibb, J.A., et al., Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature, 2001. 410(6826): p. 376–380.
Nestler, E.J., M. Barrot, and D.W. Self, Delta FosB: A sustained molecular switch for addiction. Proceedings of the National Academy of Sciences, 2001. 98(20): p. 11042.
Kumar, A., et al., Chromatin Remodeling Is a Key Mechanism Underlying Cocaine-Induced Plasticity in Striatum. Neuron, 2005. 48(2): p. 303–314.
Sekine, Y., et al., Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry, 2001. 158(8): p. 1206–1214.
Lehrmann, E., et al., Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS One, 2006. 1: p. e114.
Greenstein, R., G. Novak, and P. Seeman, Amphetamine sensitization elevates CaMKIIbeta mRNA. Synapse, 2007. 61(10): p. 827–834.
Iwata, S.I., et al., Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J Pharmacol Exp Ther, 1997. 283(3): p. 1445–1452.
Cantor-Graae, E. and J.P. Selten, Schizophrenia and migration: a meta-analysis and review. American Journal of Psychiatry, 2005. 162(1): p. 12–24.
Bourque, F., E. van der Ven, and A. Malla, A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychological Medicine, 2010: p. 1–14.
Bresnahan, M., et al., Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol, 2007. 36(4): p. 751–758.
Veling, W., et al., Ethnic density of neighborhoods and incidence of psychotic disorders among immigrants. American Journal of Psychiatry, 2008. 165(1): p. 66–73.
Boydell, J., et al., Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. Bmj, 2001. 323(7325): p. 1336–1338.
Morgan, C., et al., Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophrenia bulletin, 2010. 36(4): p. 655–664.
Selten, J.P. and E. Cantor-Graae, Social defeat: risk factor for schizophrenia? Br J Psychiatry, 2005. 187: p. 101–102.
Renthal, W., et al., Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron, 2007. 56(3): p. 517–529.
Tsankova, N.M., et al., Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci, 2006. 9(4): p. 519–525.
Cougnard, A., et al., Does normal developmental expression of psychosis combine with environmental risk to cause persistence of psychosis? A psychosis proneness-persistence model. Psychol Med, 2007: p. 1–15.
Dominguez, M.D., et al., Evidence That Onset of Clinical Psychosis Is an Outcome of Progressively More Persistent Subclinical Psychotic Experiences: An 8-Year Cohort Study. Schizophr Bull, 2009.
Baumgardner, T.L., K.E. Green, and A.L. Reiss, A behavioral neurogenetics approach to developmental disabilities: gene-brainbehavior associations. Curr Opin Neurol, 1994. 7(2): p. 172–178.
Mann, J.R., Imprinting in the germ line. Stem Cells, 2001. 19(4): p. 287–294.
Rice, K.L., I. Hormaeche, and J.D. Licht, Epigenetic regulation of normal and malignant hematopoiesis. Oncogene, 2007. 26(47): p. 6697–714.
Van Gaal, L.F., et al., Clinical endocrinology of human leptin. Int J Obes Relat Metab Disord, 1999. 23Suppl 1: p. 29–36.
Hansen, R.S., et al., The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proceedings of the National Academy of Sciences of the United States of America, 1999. 96(25): p. 14412–14417.
Klein, C.J., et al., Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nature genetics, 2011. 43(6): p. 595–600.
Sugden, C., One-carbon metabolism in psychiatric illness. Nutr Res Rev, 2006. 19(1): p. 117–136.
van der Put, N.M., et al., Folate, homocysteine and neural tube defects: an overview. Exp Biol Med (Maywood), 2001. 226(4): p. 243–270.
Zhang, H.Y., et al., Neural tube defects and disturbed maternal folate- and homocysteine-mediated one-carbon metabolism. Exp Neurol, 2008. 212(2): p. 515–521.
Pasca, S.P., et al., One carbon metabolism disturbances and the C677T MTHFR gene polymorphism in children with autism spectrum disorders. J Cell Mol Med, 2009. 13(10): p. 4229–4238.
de Jonge, R., et al., Polymorphisms in folate-related genes and risk of pediatric acute lymphoblastic leukemia. Blood, 2009. 113(10): p. 2284–2289.
Wiemels, J.L., et al., Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and risk of molecularly defined subtypes of childhood acute leukemia. Proc Natl Acad Sci U S A, 2001. 98(7): p. 4004–4009.
Kronenberg, G., M. Colla, and M. Endres, Folic acid, neurodegenerative and neuropsychiatric disease. Curr Mol Med, 2009. 9(3): p. 315–323.
Kim, Y.I., Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem, 1999. 10(2): p. 66–88.
Kim, J.M., et al., Folate, vitamin b(12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry Investig, 2008. 5(1): p. 36–40.
Levine, A.J., et al., A candidate gene study of folate-associated one carbon metabolism genes and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev, 2010. 19(7): p. 1812–1821.
Smulders, Y.M. and C.D. Stehouwer, Folate metabolism and cardiovascular disease. Semin Vasc Med, 2005. 5(2): p. 87–97.
Carmichael, S.L., et al., Hypospadias and intake of nutrients related to one-carbon metabolism. J Urol, 2009. 181(1): p. 315–321; discussion 321.
Wani, N.A., A. Hamid, and J. Kaur, Folate status in various pathophysiological conditions. IUBMB Life, 2008. 60(12): p. 834–842.
Betcheva, E.T., et al., Case-control association study of 59 candidate genes reveals the DRD2 SNP rs6277 (C957T) as the only susceptibility factor for schizophrenia in the Bulgarian population. J Hum Genet, 2009. 54(2): p. 98–107.
Feng, L.G., et al., Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: A Chinese Han population-based case-control study. Psychiatry Res, 2009. 168(3): p. 205–208.
Gaysina, D., et al., No association with the 5,10-methylenetetrahydrofolate reductase gene and major depressive disorder: results of the depression case control (DeCC) study and a meta-analysis. Am J Med Genet B Neuropsychiatr Genet, 2008. 147B(6): p. 699–706.
Gilbody, S., S. Lewis, and T. Lightfoot, Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol, 2007. 165(1): p. 1–13.
Pan, C.C., et al., Association analysis of the COMT/MTHFR genes and geriatric depression: an MRI study of the putamen. Int J Geriatr Psychiatry, 2009. 24(8): p. 847–855.
Yuan, Y.G., Z.J. Zhang, and J.J. Li, Plasma homocysteine but not MTHFR gene polymorphism is associated with geriatric depression in the Chinese population. Journal, 2010.
Yu, L., et al., No association between polymorphisms of methylenetetrahydrofolate reductase gene and schizophrenia in both Chinese and Scottish populations. Mol Psychiatry, 2004. 9(12): p. 1063–1065.
del Rio Garcia, C., et al., Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr Neurosci, 2009. 12(1): p. 13–120.
Ueland, P.M., et al., Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci, 2001. 22(4): p. 195–201.
McGuffin, P., et al., The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry, 2003. 60(5): p. 497–502.
Lichtenstein, P., et al., Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet, 2009. 373(9659): p. 234–239.
Cardno, A.G., et al., A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry, 2002. 159(4): p. 539–545.
Peerbooms, O.L., et al., Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain, behavior, and immunity, 2010.
Gonzales, M.L. and J.M. LaSalle, The role of MeCP2 in brain development and neurodevelopmental disorders. Curr Psychiatry Rep, 2010. 12(2): p. 127–134.
Amir, R.E., et al., Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet, 1999. 23(2): p. 185–188.
Klauck, S.M., et al., A mutation hot spot for nonspecific X-linked mental retardation in the MECP2 gene causes the PPM-X syndrome. Am J Hum Genet, 2002. 70(4): p. 1034–1037.
Couvert, P., et al., MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet, 2001. 10(9): p. 941–946.
Zisook, S., et al., Command hallucinations in outpatients with schizophrenia. J Clin Psychiatry, 1995. 56(10): p. 462–465.
Uddin, M., et al., Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychol Med, 2010: p. 1–11.
Uddin, M., et al., Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A, 2010. 107(20): p. 9470–9475.
Abdolmaleky, H.M., et al., Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet, 2006. 15(21): p. 3132–3145.
Grayson, D.R., et al., Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A, 2005. 102(26): p. 9341–9346.
Dempster, E.L., et al., The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC medical genetics, 2006. 7: p. 10.
Mill, J., et al., Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet, 2008. 82(3): p. 696–711.
Rosenfeld, C.S., Animal models to study environmental epigenetics. Biology of reproduction, 2010. 82(3): p. 473–488.
Veldic, M., et al., In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A, 2005. 102(6): p. 2152–2157.
Kuratomi, G., et al., Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins. Mol Psychiatry, 2007.
Mastroeni, D., et al., Epigenetic changes in Alzheimer’s disease: Decrements in DNA methylation. Neurobiol Aging, 2008.
Yoshikai, S., et al., Genomic organization of the human amyloid beta-protein precursor gene. Gene, 1990. 87(2): p. 257–263.
Tohgi, H., et al., The methylation status of cytosines in a tau gene promoter region alters with age to downregulate transcriptional activity in human cerebral cortex. Neurosci Lett, 1999. 275(2): p. 89–92.
Barrachina, M. and I. Ferrer, DNA Methylation of Alzheimer Disease and Tauopathy-Related Genes in Postmortem Brain. J Neuropathol Exp Neurol, 2009.
Wang, S.C., B. Oelze, and A. Schumacher, Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One, 2008. 3(7): p. e2698.
Silva, P.N., et al., Promoter methylation analysis of SIRT3, SMARCA5, HTERT and CDH1 genes in aging and Alzheimer’s disease. J Alzheimers Dis, 2008. 13(2): p. 173–176.
O’Connor, M.J., et al., Predictors of alcohol use prior to pregnancy recognition among township women in Cape Town, South Africa. Soc Sci Med, 2010. 72(1): p. 83–90.
Castle, D.J., et al., Does social deprivation during gestation and early life predispose to later schizophrenia? Soc Psychiatry Psychiatr Epidemiol, 1993. 28(1): p. 1–4.
Carpenter, E.M., Hox genes and spinal cord development. Dev Neurosci, 2002. 24(1): p. 24–34.
Huang, J., et al., Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. The American journal of psychiatry, 2010. 167(10): p. 1254–1263.
Argyropoulos, S.V., et al., Twins discordant for schizophrenia: psychopathology of the non-schizophrenic co-twins. Acta Psychiatrica Scandinavica, 2008. 118(3): p. 214–219.
Bjornsson, H.T., M.D. Fallin, and A.P. Feinberg, An integrated epigenetic and genetic approach to common human disease. Trends Genet, 2004. 20(8): p. 350–358.
Foley, D.L., et al., Prospects for epigenetic epidemiology. Am J Epidemiol, 2009. 169(4): p. 389–400.
Wong, C.C., et al., A longitudinal study of epigenetic variation in twins. Epigenetics: official journal of the DNA Methylation Society, 2010. 5(6): p. 516–526.
Pidsley, R. and J. Mill, Epigenetic studies of psychosis: current findings, methodological approaches, and implications for postmortem research. Biological psychiatry, 2011. 69(2): p. 146–156.
Andersen, S.L. and M.H. Teicher, Stress, sensitive periods and maturational events in adolescent depression. Trends in neurosciences, 2008. 31(4): p. 183–191.
Davey Smith, G., et al., Genetic epidemiology and public health: hope, hype, and future prospects. Lancet, 2005. 366(9495): p. 1484–1498.
McGorry, P.D., et al., Intervention in individuals at ultra-high risk for psychosis: a review and future directions. The Journal of clinical psychiatry, 2009. 70(9): p. 1206–1212.
Albert, M.S., Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A, 1996. 93(24): p. 13547–13551 *LHM: Journal available in the University Library, see the Library Catalogue for exact information *LHC: MG/SG T 0735:1963-… ISSN: 0027-8424.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is adapted from the book Chapter “Epigenetic Epidemiology”, by Bart PF Rutten & Jim van Os in the book “Epigenetic Epidemiology”, published by Springer Science + Business Media B.V., Editor Karin B. Michels, 2012, page 343-376. ISBN 978-94-007-2494-5, e-ISBN 978-94-007-2495-2, DOI 10.1007/978-94-007-2495.2 With kind permission from Springer Science+Business Media B.V.
About this article
Cite this article
Pishva, E., Kenis, G., Lesch, K.P. et al. Epigenetic epidemiology in psychiatry: A translational neuroscience perspective. Translat.Neurosci. 3, 196–212 (2012). https://doi.org/10.2478/s13380-012-0024-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s13380-012-0024-y