Abstract
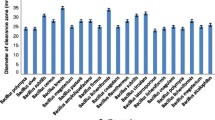
The present study aim was to isolate bacterial strains, producing protease, from soil samples of different industrial wastes and dumping areas of slaughter houses. For this, 25 bacterial isolates forming clear zones in skimmed milk agar plates as a result of casein hydrolysis were selected. One bacterial isolate showing the highest protease activity (400 U/ml) was identified as Bacillus subtilis through 16S rRNA gene sequencing. Full-length alkaline protease gene was amplified, sequenced, cloned in expression vector pT7-7, and transformed in E. coli BL21C. Over-expression of protease gene was determined in isopropyl β-d-1-thiogalactopyranoside (0.5 mM) at 37 °C for 3 h. Expressed alkaline protease was purified and 10 folds increased enzyme activity was determined as compared to the wild type B. subtilis DMA-09. The purified protease was detected on SDS-PAGE as a single band of 31 kDa and was stable at 90 °C and 85% enzyme activity was retained at 100 °C. The optimum enzyme activity was obtained at pH 10 and was enhanced in the presence of 5 mM each Mg2+ and Ca2+ while phenylmethylsulfonyl fluoride (PMSF) inhibited its activity at 0.5 mM. The expressed purified alkaline protease may find potential applications in food and biotechnological industries.



Similar content being viewed by others
Abbreviations
- SK:
-
Skimmed milk
- LBUV:
-
Luria–Bertani
- NaCl:
-
Sodium chloride
- DNA:
-
Deoxy ribonucleic acid
- IPTG:
-
Isopropyl-β-D-thiogalactopyranoside
- dNTPs:
-
deoxyribonucleotide triphosphates
- PCR:
-
Polymerase chain reaction
- NCBI:
-
National center for biotechnology information
- BLAST:
-
Basic local alignment search tool
- OD:
-
Optical density
- CM:
-
Carboxymethyl
- NaOH:
-
Sodium hydroxide
- KCl:
-
Potassium chloride
- MgCl2 :
-
Magnesium chloride
- EDTA:
-
Ethylenediaminetetraacetic acid
- PMSF:
-
Phenylmethylsulfonyl fluoride
- pCMB:
-
p-chloromercuribenzoate
- DFP:
-
Di-isopropyl fluorophosphates
- kDa:
-
Kilodalton
- ORF:
-
Open reading frame
- SDS:
-
Sodium dodecyl sulphate
- PAGE:
-
Polyacrylamide gel electrophoresis
- mL:
-
Milliliter
- M:
-
Molar
References
Abbasi-Hosseini SM, Eftekhar F, Yakhchali B, Minai-Tehrani D (2011) Cloning and enhanced expression of an extracellular alkaline protease from a soil isolate of Bacillus clausii in Bacillus subtilis. Iranian J Biotechnol 9(4): 275–280. http://www.ijbiotech.com/article_7129_45ea7dbb7127ac72c4ecbf2eae9cf6f0.pdf
Adinarayana K, Ellaiah P, Prasad DS (2003) Purification and characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. Pharma Sci Technol 4(4):1–9. https://doi.org/10.1208/pt040456
Akram M, Shafaat S, Bukhari DA, Rehman, A (2014) Characterization of a thermostable alkaline protease from Staphylococcus aureus S-2 isolated from chicken waste. Pakistan J Zool 46(4): 1125–1132. http://zsp.com.pk/pdf46/1125-1132%20(31)%20PJZ-1845-14%2015-7-14%20Manuscript%20PJZ-14%20(Page%20proof).pdf
Alikhajeh J, Khajeh K, Naderi-Mnesh M, Ranjbar B, Sajedi RH, Naderi-Mnesh H (2007) Kinetic analysis, structural studies and prediction of pKa values of Bacillus KR-8104 alpha amylase. The determinants of pH activity profile. Enzyme Microbiol Technol 41:337–345. https://doi.org/10.1016/j.enzmictec.2007.02.019
Annamalai N, Rajeswari MV, Sahu SK, Balasubramanian T (2014) Purification and characterization of solvent stable, alkaline protease from Bacillus firmus CAS 7 by microbial conversion of marine wastes and molecular mechanism underlying solvent stability. Process Biochem 49(6):1012–1019. https://doi.org/10.1016/j.procbio.2014.03.007
Asokan S, Jayanthi C (2010) Alkaline protease production by Bacillus licheniformis and Bacillus coagulans. J Cell Tissue Res 10:2119–2123. https://tcrjournals.com/tr_pastabstract.php?vid=35
Benkert P, Schwede T, Tosatto SCE (2009) QMEANclust: estimation of protein model quality by combining a composite scoring function with structural density information. BMC Struct Biol 9:35. https://doi.org/10.1186/1472-6807-9-35
Bukhari DA, Shakoori AR (2010) Isolation and molecular characterization of cry4 harbouring Bacillus thuringiensis isolates from Pakistan and mosquitocidal activity of their spores and total proteins. Pakistan J Zool 42:1–15. http://zsp.com.pk/pdf/1-15%20(1).pdf
Cappuccino JG, Sherman N (1996) Microbiology: a Laboratory Manual. Pearson education, Benjamin Cummings, San Francisco, USA. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=2429655
Chellappan S, Jasmin C, Basheer SM, Elyas KK, Bhat SG, Chandrasekaran M (2006) Production, purification and partial characterization of a novel protease from marine Engyodontium album BTMFS10 under solid state fermentation. Process Biochem 41:956–961. https://doi.org/10.1016/j.procbio.2005.10.017
Cui H, Wang L, Yu Y (2015) Production and characterization of alkaline protease from a high yielding and moderately halophilic strain of SD11 marine bacteria. J Chem 2015: 798304 (1–8). https://doi.org/10.1155/2015/798304
Deng A, Wu J, Zhang G, Wen T (2011) Molecular and structural characterization of a surfactant-stable high-alkaline protease AprB with a novel structural feature unique to subtilisin family. Biochimie 93:783–791. https://doi.org/10.1016/j.biochi.2011.01.011
Dhandapani R, Vijayaragavan R (1994) Production of a thermophilic, extracellular alkaline protease by Bacillus stearothermophilus AP-4. J Microbial Biotechnol 10:33–35. https://doi.org/10.1007/BF00357559
Donaghy JA, Mckay AM (1993) Production and properties of alkaline proteases by Aureobasidium pullulans. J Appl Bacteriol 74(6):662–666. https://doi.org/10.1111/j.1365-2672.1993.tb05200.x
Fathi Najafi M, Deobagkar D, Deobagkar D (2005) Potential application of protease isolated from Pseudomonas aeruginosa PD100. J Bacteriol 8:197–203. http://www.ejbiotechnology.info/index.php/ejbiotechnology/article/view/v8n2-5/460
Fujiwara N, Yamamoto K (1987) Production of alkaline protease in a low cost medium by alkalophilic Bacillus sp. and properties of the enzyme. J Ferment Technol 65:345–348. https://doi.org/10.1016/0385-6380(87)90098-7
Fujiwara N, Yamamoto K, Masui A (1991) Utilization of a thermostable alkaline protease from an alkalophilic thermophile for the recovery of silver from used X-ray film. J Fermen Bioengineer 72(4):306. https://doi.org/10.1016/0922-338X(91)90170-L
Gupta R, Lorenz P (2010) Bacterial alkaline protease: molecular approaches and industrial applications. Appl Microbial Biotechnol 59:15–32. https://doi.org/10.1007/s00253-002-0975-y
Horikoshi K, Akiba T (1982) Alkalophilic microorganism. A new microbial world. Japan Scientific Society Press, Tokyo. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1206263 Springer-Verlag, New York.
Ikram-ul-Haq, Mukhtar H, Daudi S, Ali S, Qadeer MA (2003) Production of proteases by a locally isolated mould culture under lab conditions. Biotechnology 2:30–36. https://doi.org/10.3923/biotech.2003.30.36
Jacobs M, Eliasson M, Uhlen M, Flock JI (1985) Cloning, sequencing and expression of subtilisin Carlsberg from Bacillus licheniformis. Nucleic Acids Res 13:8913–8926. https://doi.org/10.1093/nar/13.24.8913
Johnvesly B, Naik GR (2001) Studies of production of thermostable alkaline protease from thermophilic and alklaliphilic Bacillus sp. JB-99 in a chemical defined medium. Process Biochem 37(2):139–144. https://doi.org/10.1016/S0032-9592(01)00191-1
Jorgensen PL, Tangney M, Pedersen PE, Hastrup S, Diderichsen B, Jorgensen ST (2000) Cloning and sequencing of an alkaline protease gene from Bacillus lentus and amplification of the gene on the B. lentus chromosome by an improved technique. Appl Environ Microbiol 66:825–827. https://doi.org/10.1128/aem.66.2.825-827.2000
Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T (2009) The SWISS-MODEL repository and associated resources. Nucleic Acids Res 37:387–392. https://doi.org/10.1093/nar/gkn750
Kocher GS, Mishra S (2008) Immobilization of Bacillus circulans MTCC 7906 for enhanced production of alkaline protease under batch and packed bed fermentation conditions. Internet J Microbiol 7:2. https://ispub.com/IJMB/7/2/12651
Kronstad JW, Schnepf HE, Whiteley HR (1983) Diversity of location for the Bacillus thuringiensis crystal protein gene. J Bacteriol 154:419–428 (PMID:6833183 PMCID:pmc217475)
Kumar PKP, Mathivanan V, Karunakaran M, Renganathan S, Sreenivasan RS (2008) Studies on the effects of pH and incubation period on protease production by Bacillus spp. using groundnut cake and wheat bran. Indian J Sci Technol 1(4):1–4. DOI:https://doi.org/10.17485/ijst/2008/v1i4.2
Lin LL, Chyau CC, Hsu WH (1998) Production and properties of a raw starch degrading amylase from the thermophilic and alkalophilic Bacillus species TS-23. Biotechnol Appl Biochem 28:61–68. https://europepmc.org/article/med/9693090
Morgan FJ, Priest FG (1981) Characterization of a thermostable α-amylase from Bacillus licheniformis NCIB 6346. J Appl Bacteriol 50(1):107–114. https://doi.org/10.1111/j.1365-2672.1981.tb00875.x
Nascimento WCA, Martins MLL (2004) Production and properties of an extracellular protease from thermophilic Bacillus sp. Braz J Microbiol 35:91–96. https://doi.org/10.1590/S1517-83822004000100015
Ogino H, Yasui K, Shiotani T, Ishihara T, Ishikawa H (1995) Organic solvent-tolerant bacterium which secretes an organic solvent-stable proteolytic enzyme. Appl Environ Microbiol 61:4258–4262. https://doi.org/10.1128/AEM.61.12.4258-4262.1995
Pant G, Prakash A, Pavani JVP, Bera S, Deviram GVNS, Kumar A, Panchpuri M, Prasuna RG (2015) Production, optimization and partial purification of protease from Bacillus subtilis. J Taibah Univ Sci 9:50–55. https://doi.org/10.1016/j.jtusci.2014.04.010
Peng Y, Yang XJ, Xiao L, Zhang YZ (2004) Cloning and expression of a fibrinolytic enzyme (subtilisin DFE) gene from Bacillus amyloliquefaciens DC-4 in Bacillus subtilis. Res Microbiol 155:167–173. https://doi.org/10.1016/j.resmic.2003.10.004
Purohit MK, Singh SP (2009) Assessment of various methods for extraction of metagenomic DNA from saline habitats of coastal Gujarat (India) to explore molecular diversity. Lett Appl Microbiol 49(3):338–344. https://doi.org/10.1111/j.1472-765X.2009.02663.x
Purohit MK, Singh SP (2011) Comparative analysis of enzymatic stability and amino acid sequences of thermostable alkaline proteases from two haloalkaliphilic bacteria isolated from Coastal region of Gujarat, India. Int J Biol Macromol 49(1):103–112. https://doi.org/10.1016/j.ijbiomac.2011.04.001
Razzaq A, Shamsi S, Ali A, Ali Q, Sajjad M, Malik A, Ashraf M (2019) Microbial proteases applications. Front Bioeng Biotechnol 7:110. https://doi.org/10.3389/fbioe.2019.00110
Rehman A, Ali A, Muneer B, Shakoori AR (2007) Resistance and biosorption of mercury by bacteria isolated from industrial effluents. Pakistan J Zool 39:137–146. http://zsp.com.pk/pdf3/137-146%20(1).pdf
Sadeghi HMM, Rabbani M, Naghitorabi M (2009) Cloning of alkaline protease gene from Bacillus subtilis 168. Res Pharmaceut Sci 4:43–46. http://rps.mui.ac.ir/index.php/jrps/article/view/57
Saeki K, Ozaki K, Kobayashi T, Ito S (2007) Detergent alkaline proteases: enzymatic properties, genes and crystal structures. J Biosc Bioeng 103:501–508. https://doi.org/10.1263/jbb.103.501
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1480044
Sangeetha R, Geetha A, Arulpandi I (2008) Optimization of protease and lipase production by Bacillus pumilus SG2 isolated from an industrial effluent. Int J Microbiol 5:1–9. https://print.ispub.com/api/0/ispub-article/11512
Sareen R, Bornscheuer UT, Mishra P (2005) Cloning, functional expression and characterization of an alkaline protease from Bacillus licheniformis. Biotechnol Lett 27:1901–1907. https://doi.org/10.1007/s10529-005-3901-4
Shirai T, Suzuki A, Yamane T, AshidaT, Kobayashi T, Hitomi J, Ito S (1997) High resolution crystal structure of M-protease: phylogeny aided analysis of the high-alkaline adaptation mechanism. Protein Eng 10:627–634. https://doi.org/10.1093/protein/10.6.627
Shumi W, Towhid MH, Anwer MN (2004) Proteolytic activity of a bacterial isolate Bacillus fastidious den dorem de jong. J Biol Sci 4:370–374. https://doi.org/10.3923/jbs.2004.370.3744.370.374
Siriporn Y, Alissara R, Masaki Y (2006) Purification and characterization of alkaline protease from Bacillus megatarium isolated from Thai fish Sauce fermentation process. Sci Asia 32:377–388. https://doi.org/10.2306/scienceasia1513-1874.2006.32.377
Tang XM, Shen W, Lakay FM, Shao WL, Wang ZX, Prior BA, Zhuge J (2004) Cloning and over-expression of an alkaline protease from Bacillus licheniformis. Biotechnol Lett 26:975–979. https://doi.org/10.1023/b:bile.0000030042.91094.38
Author information
Authors and Affiliations
Contributions
DAB designed, performed experiments, and analyzed the results. AB helped in conducting experiments. AR supervised the study and helped in manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 171 KB)
Rights and permissions
About this article
Cite this article
Bukhari, D.A., Barkat, A. & Rehman, A. Expression, purification, and molecular characterization of a full-length thermostable alkaline protease gene from Bacillus subtilis DMA-09. Biologia 76, 741–750 (2021). https://doi.org/10.2478/s11756-020-00608-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00608-6