Abstract
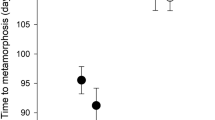
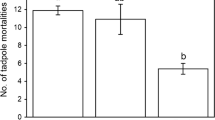
Changes in environmental conditions can induce organisms to alter their morphology, behavior and life history. Predation is an important factor in many aquatic communities and can strongly select for anti-predator responses. In the present work, we examined the responses in morphology, growth rate and development rate of Elachistocleis bicolor tadpoles raised in the presence of chemical cues from two different predators: a water bug (Belostoma elongatum) and a fish (Moenkausia dichroura). The experiment was performed in microcosm conditions. The experimental design consisted of three treatments: chemical cues from fish, cues from water bugs and a control group. Each treatment was replicated 30 times. Each container held a single larva. The main results were: (1) there were significant differences in body depth between the predator treatments (fish vs. water bug) and between the control group and the water bug treatment, (2) there were significant differences in tail depth between predator treatments (fish vs. water bug) and between the control group and the fish treatment, (3) there were no significant differences in the growth rate and developmental rate among the treatments. Our results suggest that the presence of predaceous fish and water bugs cause different effects on tadpole morphology. In the presence of water bugs, tadpoles decreased body depth, whereas in the presence of fish tadpoles increased tail depth. These responses could be related to the way in which predators capture their prey. Predator chemical cues did not have any detectable effect on the growth rate and development rate of E. bicolor tadpoles.
Similar content being viewed by others
References
Addinsoft. 2006. XLSTAT version 7. 5. for Excel interface. Addinsoft, U.K.
Benard M.F. 2006. Survival trade-offs between two predator induced phenotypes in Pacific treefrogs (Pseudacris regilla). Ecology 87(2): 340–346. DOI: 10.1890/05-0381
Benard M.F. 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Ann. Rev. Ecol. Evol. Syst. 35: 651–673. DOI: 10.1146/annurev.ecolsys.35.021004.112426
Blaustein L. 1999. Oviposition site selection in response to risk of predation: evidence from aquatic habitats and consequences for population dynamics and community structure, pp. 442–456. In: Wasser S.P. (ed.), Evolutionary Theory and Processes: Modern Perspectives, Kluwer Academic Publishers, Netherlands. ISBN: 0792355180
Blaustein L., Friedman F. & Fahima T. 1996. Larval Salamandra drive temporary pool community dynamics: evidence from an artificial pool experiment. Oikos 76(2): 392–402. DOI: 10.2307/3546211
Darlington R.B. & Smulders T.V. 2001. Problems with residuals analysis. Anim. Behav. 62: 599–602 Part 3. DOI: 10.1006/anbe.2001.1806
Gómez V.I. & Kehr A.I. 2011. Morphological and developmental responses of anuran larvae (Physalaemus albonotatus) to chemical cues from predators Moenkausia dichroura (Characiformes: Characidae) and Belostoma elongatum (Hemiptera: Belostomatidae). Zool. Stud. 50(2): 203–210.
Gomulkiewicz R. & Kirkpatrick M. 1992. Quantitative genetics and the evolution of reaction norms. Evolution 46(2): 390–411. DOI: 10.2307/2409860
Gosner K.L. 1960. A simplified table for staging anurans embryos and larvae with notes of identification. Herpetologica 16(3): 183–190.
Hews D.K. 1988. Alarm response in larval western toads, Bufo boreas: release of larval chemicals by a natural predator and its effect on predator capture efficiency. Anim. Behav. 36(1): 125–133. DOI: 10.1016/S0003-3472(88)80255-0
Holling C.S. 1964. The analysis of complex population processes. Canad. Entomol. 96(1–2): 335–347. DOI: 10.4039/Ent963-4
Kiesecker J.M., Chivers D.P., Anderson M. & Blaustein A.R. 2002. Effect of predator diet on life history shifts of red-legged frogs, Rana aurora. J. Chem. Ecol. 28(5): 1007–1015. DOI: 10.1023/A:1015261801900
Kishida O., Mizuta Y. & Nishimura K. 2006. Reciprocal phenotypic plasticity in a predator-prey interaction between larval amphibians. Ecology 87(6): 1599–1604. DOI: 10.1890/0012-9658(2006)87[1599:RPPIAP]2.0.CO;2
Kishida O. & Nishimura K. 2005. Multiple inducible defenses against multiple predators in the anuran tadpole, Rana pirica. Evol. Ecol. Res. 7(4): 619–631.
Lardner B. 2000. Morphological and life history response to predators in larvae of seven anurans. Oikos 88(1): 169–180. DOI: 10.1034/j.1600-0706.2000.880119.x
Laurila A., Pakkasmaa S. & Merila J. 2006. Population divergence in growth rate and antipredator defenses in Rana arvalis. Oecologia 147(4): 585–595. DOI: 10.1007/s00442-005-0301-3
Laurila A., Pakkasmaa S. & Merila J. 2004. Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defenses. Oikos 107(1): 90–99. DOI: 10.1111/j.0030-1299.2004.13126.x
Lima S.L. & Dill L.M. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68(4): 619–640. DOI: 10.1139/z90-092
McCollum S.A. & Van Buskirk J. 1996. Costs and benefits of a predator-induced polyphenism in the Gray treefrog Hyla chrysoscelis. Evolution 50(2): 583–593. DOI: 10.2307/2410833
Moran N.A. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139(5): 971–989.
Peacor S.D. & Werner E.E. 2004. Context dependent of nonlethal effect of a predator on prey growth. Isr. J. Zool. 50(2–3): 139–157. DOI: 10.1560/KPRR-X1C3-5NHE-QV2N
Relyea R.A. 2007. Getting out alive: how predators affect the decision to metamorphose. Oecologia 152(3): 389–400. DOI: 10.1007/s00442-007-0675-5
Relyea R.A. 2003. Predators come and predators go: The reversibility of predator-induced traits. Ecology 84(7): 1840–1848. DOI: 10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2
Relyea R.A. 2001a. The relationship between predation risk and antipredator responses in larval anurans. Ecology 82(2): 541–554. DOI: 10.1890/0012-9658(2001)082[0541:TRBPRA] 2.0.CO;2
Relyea R.A. 2001b. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82(2): 523–540. DOI: 10.1890/0012-9658(2001)082[0523: MABPOL]2.0.CO;2
Relyea R.A. & Werner E.E. 2000. Morphological plasticity in four larval anurans distributed along an environmental gradient. Copeia 2000(1): 178–190 DOI: 10.1643/0045-8511(2000)2000[0178:MPIFLA]2.0.CO;2)
Schlichting C.D. & Pigliucci M. 1998. Phenotypic Evolution: A Reaction Norm Perspective. Sinauer Associates Publishers, Sunderland, MA, 340 pp. ISBN: 0-87893-799-4
Skelly D.K. 2001. Distribution of pond-breeding anurans: an overview of mechanisms. Isr. J. Zool. 47(4): 313–332. DOI: 10.1560/BVT1-LUYF-2XG6-B007
SPSS. 1997. SYSTAT 7.5 for Window. SPSS Inc. Chicago-USA.
Teplitsky C., Plénet S. & Joly P. 2003. Tadpoles responses to the risk of fish introduction. Oecologia 134(2): 270–277. DOI: 10.1007/s00442-002-1106-2
Teplitsky C., Plénet S. & Joly P. 2004. Hierarchical responses to tadpoles to multiple predators. Ecology 85(10): 2888–2894. DOI: 10.1890/03-3043
Teplitsky C., Plénet S., Lena J.P., Mermet N., Malet E. & Joly P. 2005. Escape behavior and ultimate causes of specific induces defenses in an anuran tadpole. J. Evol. Biol. 18(1): 180–190. DOI: 10.1111/j.1420-9101.2004.00790.x
Tollrian R. & Harvell D. (eds) 1999. The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, NJ, 383 pp. ISBN: 0691004943, 9780691004945
Van Buskirk J. 2000. The cost of an inducible defense in anuran larvae. Ecology 81(10): 2813–2821. DOI: 10.1890/0012-9658(2000)081[2813:TCOAID]2.0.CO;2
Van Buskirk J. 2001. Specific induced responses to different predator species in anuran larvae. J. Evol. Biol. 14: 482–489. DOI: 10.1046/j.1420-9101.2001.00282.x
Van Buskirk J. 2002. Phenotypic lability and the evolution of predator-induced plasticity in tadpoles. Evolution 56(2): 361–370. PMID: 11926504
Van Buskirk J., McCollum S.A. & Werner E.E. 1997. Natural selection for environmentally induced phenotypes in tadpoles. Evolution 51(6): 1983–1992.
Van Buskirk J. & Relyea R.A. 1998. Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol. J. Linn. Soc. 65(3): 301–328. DOI: 10.1111/j.1095-8312.1998.tb01144.x
Wassersug R.J. 1989 Locomotion in amphibian larvae (or “Why aren’t tadpoles built like fishes?”). Amer. Zool. 29(1): 65–84. DOI: 10.1093/icb/29.1.65
Werner E.E. 1986. Amphibian metamorphosis: growth rate, predation risk, and optimal size at transformation. Am. Nat. 128(3): 319–341.
Werner E.E. & McPeek M.A. 1994. Direct and indirect effect of predators on two anuran species along an environmental gradient. Ecology 75(5): 1368–1382. DOI: 10.2307/1937461
Wilbur H.M. 1997. Experimental ecology of food webs: complex systems in temporary ponds. Ecology 78(8): 2279–2302. DOI: 10.1890/0012-9658(1997)078[2279:EEOFWC]2.0.CO;2
Williams J.D. & Gudynas E. 1987. Descripción de la larva de Elachistocleis bicolor (Valenciennes 1838) (Anura: Microhylidae). Amphibia-Reptilia 8(3): 225–229. DOI: 10.1163/156853887X00261
Wojdak J.M. & Trexler D.C. 2010. The influence of temporally variable predation risk on indirect interactions in an aquatic food chain. Ecol. Res. 25(2): 327–335. DOI: 10.1007/s11284-009-0664-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez, V.I., Kehr, A.I. The effect of chemical signal of predatory fish and water bug on the morphology and development of Elachistocleis bicolor tadpoles (Anura: Microhylidae). Biologia 67, 1001–1006 (2012). https://doi.org/10.2478/s11756-012-0082-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-012-0082-1