Abstract
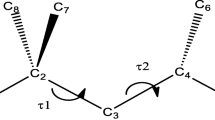
The current work is a study of the conformational space of the non-ionic N-formylmethionine molecule around its seven structurally significant internal backbone torsional angles at B3LYP/6-31++G(d,p) levels of theory in the gaseous phase. The potential energy surface exploration reveals that a total of 432 different conformers would result if all the possible combinations of the internal rotations were to be considered. A set of twelve conformers of the N-formylmethionine molecule are then further analysed in terms of their relative stabilities, theoretically predicted harmonic vibrational frequencies, HOMO-LUMO energy gaps, ESP charges, rotational constants and dipole moments calculated using MP2/6-31++G(d,p) and B3LYP/6-311++G(d,p) levels. The calculated relative energy-range of the conformers at the MP2 level is 11.08 kcal mol−1 (1 kcal = 4.1868 kJ), whereas the same obtained at the B3LYP level is 10.02 kcal mol−1. The results of this study provide a good account of the role of four types of intramolecular H-bonds, namely O…H—O, O…H—N, O…H—C and N…H—C, in influencing the energies of the conformers as well as their conformational and vibrational spectroscopic aspects. The relative stability order of the conformers appears to depend on the level of theory used while the vibrational frequencies calculated at the B3LYP level are in better agreement with the experimental values.
Similar content being viewed by others
References
Andersson, M. P., & Uvdal, P. (2005). New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6-311+G(d,p). The Journal of Physical Chemistry A, 109, 2937–2941. DOI: 10.1021/jp045733a.
Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics, 98, 5648–5652. DOI: 10.1063/1.464913.
Brovarets, O. O., & Hovorun, D. M. (2014). Can tautomerization of the A·T Watson-Crick base pair via double proton transfer provoke point mutations during DNA replication? A comprehensive QM and QTAIM analysis. Journal of Biomolecular Structure and Dynamics, 32, 127–154. DOI: 10.1080/07391102.2012.755795.
Chen, M., Huang, Z., & Lin, Z. (2005). Ab initio studies of gas phase asparagine conformers. Journal of Molecular Structure: THEOCHEM, 719, 153–158. DOI: 10.1016/j.theochem.2005.01.019.
Clark, B. F. C., & Marcker, K. A. (1966). N-Formyl-methionylsribonucleic acid and chain initiation in protein biosynthesis: Polypeptide synthesis directed by a bacteriophage ribonucleic acid in a cell-free system. Nature, 211, 378–380. DOI: 10.1038/211378a0.
Coldren, C. D., Hellinga, H. W., & Caradonna, J. P. (1997). The rational design and construction of a cuboidal iron-sulfur protein. Proceedings of the National Academy of Sciences of the United States of America, 94, 6635–6640. DOI: 10.1073/pnas.94.13.6635.
Czinki, E., & Császár, A. G. (2003). Conformers of gaseous proline. Chemistry — A European Journal, 9, 1008–1019. DOI: 10.1002/chem.200390103.
Das, G. (2013a). Investigations of dipeptide structures containing pyrrolysine as N-terminal residues: a DFT study in gas and aqueous phase. Journal of Molecular Modeling, 19, 1901–1911. DOI: 10.1007/s00894-013-1754-7.
Das, G. (2013b). Zwitterionic conformers of pyrrolysine and their interactions with metal ions—a theoretical study. Journal of Molecular Modeling, 19, 2981–2991. DOI: 10.1007/s00894-013-1829-5.
Das, G., & Lyngdoh, R. H. D. (2014). Configuration of wobble base pairs having pyrimidines as anticodon wobble bases: significance for codon degeneracy. Journal of Biomolecular Structure and Dynamics, 32, 1500–1520. DOI: 10.1080/07391102.2013.824822.
Das, G., & Mandal, S. (2013). DFT studies on the intrinsic conformational properties of non-ionic pyrrolysine in gas phase. Journal of Molecular Modeling, 19, 1695–1704. DOI: 10.1007/s00894-012-1740-5.
DeGrado, W. F., Summa, C. M., Pavone, V., Nastri, F., & Lombardi, A. (1999). De novo design and structural characterization of proteins and metalloproteins. Annual Review of Biochemistry, 68, 779–819. DOI: 10.1146/annurev.biochem.68.1.779.
De Proft, F., Martin, J. M. L., & Geerlings, P. (1996). On the performance of density functional methods for describing atomic populations, dipole moments and infrared intensities. Chemical Physics Letters, 250, 393–401. DOI: 10.1016/0009-2614(96)00057-7.
Dimitrova, Y. (2004). Structure, stability and vibrational spectrum of the hydrogen-bonded complex between HNO3 and H2O. Ab initio and DFT studies. Spectrochimica Acta Part A, 60, 1–8. DOI: 10.1016/s1386-1425(03)00180-x.
Foresman, J. B., & Frisch, A. (1996). Exploring chemistry with electronic structure methods (2nd ed.). Pittsburgh, PA, USA: Gaussian, Inc.
Freeman, F., & Le, K. T. (2003). A computational study of conformations and conformers of 1,3-dithiane (1,3-dithiacyclohexane). The Journal of Physical Chemistry A, 107, 2908–2918. DOI: 10.1021/jp0138633.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, J. A., Jr., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., & Pople, J. A. (2003). Gaussian 03, Revision B.04 [computer software]. Pittsburgh, PA, USA: Gaussian, Inc.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A., Jr., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, N. J., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., & Fox, D. J. (2010) Gaussian 09, Revision B.01 [computer software]. Wallingford, CT, USA: Gaussian, Inc.
Gronert, S., & O’Hair, R. A. J. (1995). Ab initio studies of amino acid conformations 1. The conformers of alanine, serine, and cysteine. Journal of the American Chemical Society, 117, 2071–2081. DOI: 10.1021/ja00112a022.
Hehre, W. J., Radom, L., von Ragué Schleyer, P., & Pople, J. A. (1986). Ab initio molecular orbital theory. New York, NY, USA: Wiley.
Hernández, B., Pflüger, F., Adenier, A., Kruglik, S. G., & Ghomi, M. (2011). Side chain flexibility and protonation states of sulfur atom containing amino acids. Physical Chemistry Chemical Physics, 13, 17284–17294. DOI: 10.1039/c1cp21054h.
Huang, Z., & Lin, Z. (2005). Detailed ab initio studies of the conformers and conformational distributions of gaseous tryptophan. The Journal of Physical Chemistry A, 109, 2656–2659. DOI: 10.1021/jp0461201.
Kaur, D., Sharma, P., Bharatam, P. V., & Kaur, M. (2008). Understanding selenocysteine through conformational analysis, proton affinities, acidities and bond dissociation energies. International Journal of Quantum Chemistry, 108, 983–991. DOI: 10.1002/qua.21556.
Khorana, H. G., Büuchi, H., Ghosh, H., Gupta, N., Jacob, T. M., Kössel, H., Morgan, R., Narang, S. A., Ohtsuka, E., & Wells, R. D. (1966). Polynucleotide synthesis and the genetic code. Cold Spring Harbor Symposia on Quantitative Biology, 31, 39–49. DOI: 10.1101/sqb.1966.031.01.010.
Lambie, B., Ramaekers, R., & Maes, G. (2004). Conformational behavior of serine: An experimental matrix-isolation FT-IR and theoretical DFT(B3LYP)/6-31++G** study. The Journal of Physical Chemistry A, 108, 10426–10433. DOI: 10.1021/jp047192v.
Láng, A., Csizmadia, I. G., & Perczel, A. (2005). Peptide models XLV: Conformational properties of N-formyl-l-methioninamide and its relevance to methionine in proteins. Proteins: Structure, Function, and Bioinformatics, 58, 571–588. DOI: 10.1002/prot.20307.
Lee, C., Yang, W., & Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37, 785–789. DOI: 10.1103/PhysRevB.37.785.
Lee, S. Y., & Boo, B. H. (1996). Density functional theory study of vibrational spectra of fluorene. The Journal of Physical Chemistry, 100, 8782–8785. DOI: 10.1021/jp960020g.
Lee, K. T., Sung, J., Lee, K. J., Park, Y. D., & Kim, S. K. (2002). Conformation-dependent ionization energies of Lphenylalanine. Angewandte Chemie International Edition, 41, 4114–4117. DOI: 10.1002/1521-3773(20021104)41:21<4114::AID-ANIE4114>3.0.CO;2-M.
Lesarri, A., Sánchez, R., Cocinero, E. J., López, J. C., & Alonso, J. L. (2005). Coded amino acids in gas phase: The shape of isoleucine. Journal of the American Chemical Society, 127, 12952–12956. DOI: 10.1021/ja0528073.
Linder, R., Seefeld, K., Vavra, A., & Kleinermanns, K. (2008). Gas phase infrared spectra of nonaromatic amino acids. Chemical Physics Letters, 453, 1–6. DOI: 10.1016/j.cplett.2007.12.069.
Lu, Y. (2005). Design and engineering of metalloproteins containing unnatural amino acids or non-native metalcontaining cofactors. Current Opinion in Chemical Biology, 9, 118–126. DOI: 10.1016/j.cbpa.2005.02.017.
Maksić, Z. B., & Kovačević, B. (1999). Neutral vs. zwitterionic form of arginine-an ab initio study. Journal of the Chemical Society, Perkin Transactions 2, 1999, 2623–2629. DOI: 10.1039/a902404b.
Mandal, S., & Das, G. (2013). Structure of dipeptides having N-terminal selenocysteine residues: a DFT study in gas and aqueous phase. Journal of Molecular Modeling, 19, 2613–2623. DOI: 10.1007/s00894-013-1808-x.
McCarthy, K. F., & Lovenberg, W. (1970). N-formylmethionine: the N-terminus of Clostridium pasteurianum rubredoxin. Biochemical and Biophysical Research Communications, 40, 1053–1057. DOI: 10.1016/0006-291x(70)90900-9.
Nakamoto, T., & Kolakofsky, D. (1966). A possible mechanism for initiation of protein synthesis. Proceedings of the National Academy of Sciences of the United States of America, 55, 606–613. DOI: 10.1073/pnas.55.3.606.
Noei, M., Salari, A. A., Baei, M. T., Hajizadeh, F., Asl, J. K., & Taghartapeh, M. R. (2014). Theoretical study of fMet-tRNA and fAla-tRNA structures by using quantum calculation. Arabian Journal of Chemistry, in press. DOI: 10.1016/j.arabjc.2011.11.010.
Osawa, S., Jukes, T. H., Watanabe, K., & Muto, A. (1992). Recent evidence for evolution of the genetic code. Microbiological Reviews, 56, 229–264.
Peterson, J. R., Bickford, L. C., Morgan, D., Kim, A. S., Ouerfelli, O., Kirschner, M. W., & Rosen, M. K. (2004). Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nature Structural & Molecular Biology, 11, 747–755. DOI: 10.1038/nsmb796.
Plaxco, K. W., & Groß, M. (1997). The importance of being unfolded. Nature, 386, 657–659. DOI: 10.1038/386657a0.
Rak, J., Skurski, P., Simons, J., & Gutowski, M. (2001). Lowenergy tautomers and conformers of neutral and protonated arginine. Journal of the American Chemical Society, 123, 11695–11707. DOI: 10.1021/ja011357l.
Shirazian, S., & Gronert, S. (1997). The gas-phase conformations of valine: an ab initio study. Journal of Molecular Structure (Theochem), 397, 107–112. DOI: 10.1016/s0166-1280(96)04939-1.
Stepanian, S. G., Reva, I. D., Radchenko, E. D., & Adamowicz, L. (1998a). Conformational behavior of α-alanine. Matrixisolation infrared and theoretical DFT and ab initio study. The Journal of Physical Chemistry A, 102, 4623–4629. DOI: 10.1021/jp973479z.
Stepanian, S. G., Reva, I. D., Radchenko, E. D., Rosado, M. T. S., Duarte, M. L. T. S., Fausto, R., & Adamowicz, L. (1998b). Matrix-isolation infrared and theoretical studies of the glycine conformers. The Journal of Physical Chemistry A, 102, 1041–1054. DOI: 10.1021/jp973397a.
Sundararajan, T. A., & Thach, R. E. (1966). Role of the formylmethionine codon AUG in phasing translation of synthetic messenger RNA. Journal of Molecular Biology, 19, 74–90. DOI: 10.1016/s0022-2836(66)80051-7.
Szidarovszky, T., Czakó, G., & Császár, A. G. (2009). Conformers of gaseous threonine. Molecular Physics, 107, 761–775. DOI: 10.1080/00268970802616350.
Tehrani, Z. A., Tavasoli, E., & Fattahi, A. (2010). Conformational behavior and potential energy profile of gaseous histidine. Journal of Molecular Structure: THEOCHEM, 960, 73–85. DOI: 10.1016/j.theochem.2010.08.025.
Uversky, V. N., Gillespie, J. R., & Fink, A. L. (2000). Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins: Structure, Function, and Genetics, 41, 415–427. DOI: 10.1002/1097-0134(20001115)41:3〈415::AIDPROT130〉3.0.CO;2-7.
Xie, J., Liu, W., & Schultz, P. G. (2007). A genetically encoded bidentate, metal-binding amino acid. Angewandte Chemie International Edition, 46, 9239–9242. DOI: 10.1002/anie.200703397.
Yurenko, Y. P., Zhurakivsky, R. O., Samijlenko, S. P., & Hovorun, D. M. (2011). Intramolecular CH…O hydrogen bonds in the AI and BI DNA-like conformers of canonical nucleosides and their Watson-Crick pairs. Quantum chemical and AIM analysis. Journal of Biomolecular Structure and Dynamics, 29, 51–6 DOI: 10.1080/07391102.2011.10507374.
Zhang, M., & Lin, Z. (2006). Ab initio studies of the conformers and conformational distribution of the gaseous hydroxyamino acid threonine. Journal of Molecular Structure: THEOCHEM, 760, 159–166. DOI: 10.1016/j.theochem.2005.12.008.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Das, G., Mandal, S. Ab initio- and density-functional studies of conformational behaviour of N-formylmethionine in gaseous phase. Chem. Pap. 68, 1608–1620 (2014). https://doi.org/10.2478/s11696-014-0614-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0614-y