Abstract
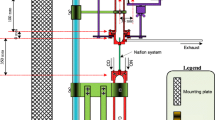
A method for the rapid and sensitive determination of peroxyacetyl nitrate (PAN) in air based on a chemiluminescence reaction with an alkaline solution of luminol in the chemiluminescence aerosol detector is described. The PAN is chromatographically separated from nitrogen dioxide and ozone in a packed column filled with 5 % OV-1 on Chromosorb 30/60 and the eluted PAN is detected via the direct reaction with the luminol solution consisting of 0.002 mol L−1 luminol, 1 vol. % Brij-35 and 0.1 mol L−1 KOH. The limit of detection is 14.9 ng m−3 (3 ppt) of PAN. Alternatively, the PAN after separation is thermally converted to NO2 which is detected by the chemiluminescence reaction with a solution consisting of 0.002 mol L−1 luminol, 0.5 mol L−1 KOH, 0.2 mol L−1 Na2SO3, 0.1 mol L−1 KI, 0.05 mol L−1 EDTA and 0.5 vol. % triton X-100. The alternative approach affords the simultaneous determination of PAN and NO2. The limit of detection is 50 ppt of PAN and 50 ppt of NO2. The time resolution is 3 min. The method was applied to the measurement of ambient peroxyacetyl nitrate in air.
Similar content being viewed by others
References
Blanchard, P., Shepson, P. B., So, K. W., Schiff, H. I., Bottenheim, J. W., Gallant, A. J., Drummond, J. W., & Wong, P. (1990). A comparison of calibration and measurement techniques for gas chromatographic determination of atmospheric peroxyacetyl nitrate (PAN). Atmospheric Environment. Part A. General Topics, 24, 2839–2846. DOI: 10.1016/0960-1686(90)90171-i.
Blanchard, P., Shepson, P. B., Schiff, H. I., & Drummond, J. W. (1993). Development of a gas chromatograph for trace level measurement of peroxyacetyl nitrate using chemical amplification. Analytical Chemistry, 65, 2472–2477. DOI: 10.1021/ac00066a012.
Bružeňák, L. (2005). Aplikace chemiluminiscenční detekce při stanovení plynných polutantů ve vzduchu. Masters thesis. Brno, Czech Republic: University of Technology (in Czech)
Burkhardt, M. R., Maniga, N. I., Stedman, D. H., & Paur, R. J. (1988). Gas chromatographic method for measuring nitrogen dioxide and peroxyacetyl nitrate in air without compressed gas cylinders. Analytical Chemistry, 60, 816–819. DOI: 10.1021/ac00159a017.
Carter, A. G., & Travers, M. W. (1937). On the thermal decomposition of methyl nitrite. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 158, 495–498. DOI: 10.1098/rspa.1937.0035.
Ciccioli, P., Possanzini, M., Di Palo, V., & Cecinato, A. (1992). Dynamic calibration of peroxyacetyl nitrate (PAN) analyzers by annular denuder and ion-chromatographic techniques. Atmospheric Environment. Part A. General Topics, 26, 1513–1518. DOI: 10.1016/0960-1686(92)90135-8.
Clemitshaw, K. (2004). A review of instrumentation and measurement techniques for ground-based and airborne field studies of gas-phase tropospheric chemistry. Critical Reviews in Environmental Science and Technology, 34, 1–108. DOI: 10.1080/10643380490265117.
Day, D. A., Wooldridge, P. J., Dillon, M. B., Thornton, J. A., & Cohen, R. C. (2002). A thermal dissociation laserinduced fluorescence instrument for in situ detection of NO2, peroxy nitrates, alkyl nitrates and HNO3. Journal of Geophysical Research: Atmospheres, 107, 4046. DOI: 10.1029/2001jd000779.
Danalatos, D., & Glavas, S. (1997). Improvement in the use of capillary columns for ambient air peroxyacetyl nitrate monitoring. Journal of Chromatography A, 786, 361–365. DOI: 10.1016/s0021-9673(97)00615-8.
De Santis, F., Allegrini, I., Di Filippo, P., & Pasella, D. (1996). Simultaneous determination of nitrogen dioxide and peroxyacetyl nitrate in ambient atmosphere by carbon-coated annular diffusion denuder. Atmospheric Environment, 30, 2637–2645. DOI: 10.1016/1352-2310(95)00296-0.
Finlayson-Pitts, B. J., & Pitts, J. N. (2000). Chemistry of the upper and lower atmosphere. Theory, experiments, and applications. San Diego, CA, USA: Academic Press.
Gaffney, J. S., Fajer, R., & Senum, G. I. (1984). An improved procedure for high purity gaseous peroxyacyl nitrate production: Use of heavy lipid solvents. Atmospheric Environment, 18, 215–218. DOI: 10.1016/0004-6981(84)90245-2.
Gaffney, J. S., Marley, N. A., & Prestbo, E. W. (1989). Peroxyacyl nitrates (PANS): Their physical and chemical properties. In O. Hutzinger (Ed.), The handbook of environmental chemistry (Vol. 4, Part B, pp. 1–38). Berlin, Germany: Springer.
Gaffney, J. S., Bornick, R. M., Chen, Y. H., & Marley, N. A. (1998). Capillary gas chromatographic analysis of nitrogen dioxide and PANs with luminol chemiluminescent detection. Atmospheric Environment, 32, 1445–1454. DOI: 10.1016/s1352-2310(97)00098-8.
Grosjean, D., & Harrison, J. (1985). Peroxyacetyl nitrate: Comparison of alkaline hydrolysis and chemiluminescence methods. Environmental Science & Technology, 19, 749–752. DOI: 10.1021/es00138a017.
Grosjean, D., Parmar, S. S., & Williams, E. L. (1991). Time-averaged measurements of peroxyacetyl nitrate. Environmental Science & Technology, 25, 1864–1867. DOI: 10.1021/es00023a003.
Gregory, G. L., Hoell, J. M., Ridley, B. A., Singh, H. B., Gandrud, B., Salas, L. J., & Shetter, J. (1990). An intercomparsion of airborne PAN measurements. Journal of Geophysical Research: Atmospheres, 95, 10077–10087. DOI: 10.1029/jd095id07p10077.
Güsten, H. (1986). Formation, transport and control of photochemical smog. In O. Hutzinger (Ed.), The handbook of environmental chemistry (Vol. 4, Part B, pp. 53–105). Berlin, Germany: Springer.
Hansel, A., & Wisthaler, A. (2000). A method for real-time detection of PAN, PPN and MPAN in ambient air. Geophysical Research Letters, 27, 895–898. DOI: 10.1029/1999gl010989.
Hao, C. S., Shepson, P. B., Drummond, J. W., & Muthuramu, K. (1994). Gas-chromatographic detector for selective and sensitive detection of atmospheric organic nitrates. Analytical Chemistry, 66, 3737–3743. DOI: 10.1021/ac00093a032.
Hastie, D. R., Gray, J., Langford, V. S., Maclagan, R. G. A. R., Milligan, D. B., & McEwan, M. J. (2010). Real-time measurement of peroxyacetyl nitrate using selected ion flow tube mass spectrometry. Rapid Communications in Mass Spectrometry, 24, 343–348. DOI: 10.1002/rcm.4400.
Holdren, M. W., & Spicer, C. W. (1984). Field compatible calibration procedure for peroxyacetyl nitrate. Environmental Science & Technology, 18, 113–116. DOI: 10.1021/es00120a013.
Kleindienst, T. E., Shepson, P. B., Edney, E. O., & Claxton, L. D. (1985). Peroxyacetyl nitrate: Measurement of its mutagenic activity using the Salmonella/mammalian microsome reversion assay. Mutation Research/Genetic Toxicology, 157, 123–128. DOI: 10.1016/0165-1218(85)90106-5.
Lee, G. W., Choi, H. S., Lee, T. Y., Choi, J. S., Park, J. S., & Ahn, J. Y. (2012). Variations of regional background peroxyacetyl nitrate in marine boundary layer over Baengyeong Island, South Korea. Atmospheric Environment, 61, 533–541. DOI: 10.1016/j.atmosenv.2012.07.075.
Marley, N. A., Gaffney, J. S., White, R. V., Rodriguez-Cuadra, L., Herndon, S. E., Dunlea, E., Volkamer, R. M., Molina, L. T., & Molina, M. J. (2004). Fast gas chromatography with luminal chemiluminescence detection for the simultaneous de termination of nitrogen dioxide and peroxyacetyl nitrate in the atmosphere. Review of Scientific Instruments, 75, 4595–4605. DOI: 10.1063/1.1805271.
Mineshos, G., Roumelis, N., & Glavas, S. (1991). Determinaton of peroxyacetyl nitrate, peroxypropionyl nitrate and alkyl nitrates of atmospheric importance using capillary columns. Journal of Chromatography A, 541, 99–108. DOI: 10.1016/s0021-9673(01)95987-4.
Mikuška, P., & Večeřa, Z. (1992). Determination of nitrogen dioxide with a chemiluminescent aerosol detector. Analytical Chemistry, 64, 2187–2191. DOI: 10.1021/ac00042a029.
Mikuška, P., Štikarovský, J., & Zdráhal, Z. (1993). Ozone source. Chemické Listy, 87, 441–443.
Mikuška, P., Večeřa, Z., & Zdráhal, Z. (1995). Flow-injection chemiluminescence determination of ultra low concentrations of nitrite in water. Analytica Chimica Acta, 316, 261–268. DOI: 10.1016/0003-2670(95)00369-b.
Mikuška, P., & Večeřa, Z. (1998). Application of gallic acid and xanthene dyes for determination of ozone in air with a chemiluminescence aerosol detector. Analytica Chimica Acta, 374, 297–302. DOI: 10.1016/s0003-2670(98)00453-x.
Mikuška, P., & Večeřa, Z. (2000). Effect of complexones and tensides on selectivity of nitrogen dioxide determination in air with a chemiluminiscence aerosol detector. Analytica Chimica Acta, 410, 159–165. DOI: 10.1016/s0003-2670(00)00710-8.
Mikuška, P., Motyka, K., & Večeřa, Z. (2008). Determination of nitrous acid in air using wet effluent diffusion denuder-FIA technique. Talanta, 77, 635–641. DOI: 10.1016/j.talanta.2008.07.008.
Müller, K. P., & Rudolph, J. (1989). An automated technique for the measurement of peroxyacetylnitrate in ambient air at ppb and ppt levels.International Journal of Environmental Analytical Chemistry, 37, 253–262. DOI: 10.1080/03067318908026902.
Nikitas, C., Clemitshaw, K. C., Oram, D. E., & Penkett, S. A. (1997). Measurements of PAN in the polluted boundary layer and free troposphere using a luminol-NO2 detector combined with a thermal converter. Journal of Atmospheric Chemistry, 28, 339–359. DOI: 10.1023/a:1005898017520.
Simon, P. K., Dasgupta, P. K., & Večeřa, Z. (1991). Wet effluent denuder coupled liquid/ion chromatography systems. Analytical Chemistry, 63, 1237–1242. DOI: 10.1021/ac00013a011.
Slusher, D. L., Huey, L. G., Tanner, D. J., Flocke, F. M., & Roberts, J. M. (2004). A thermal dissociation-chemical ionization mass spectrometry (TD-CIMS) technique for the simultaneous measurement of peroxyacyl nitrates and dinitrogen pentoxide. Journal of Geophysical Research, 109, D19315. DOI: 10.1029/2004jd004670.
Pérez, I. M., Wooldridge, P. J., & Cohen, R. C. (2007). Laboratory evaluation of a novel thermal dissociation chemiluminescence method for in situ detection of nitrous acid. Atmospheric Environment, 41, 3993–4001. DOI: 10.1016/j.atmosenv.2007.01.060.
Pitts, J. N., Fuhr, H., Gaffney, J. S., & Peters, J. W. (1973). Chemiluminiscent reactions of peroxyacetyl nitrate and ozone with triethylamine. Possible atmospheric monitor for peroxyacetyl nitrate. Environmental Science & Technology, 7, 550–552. DOI: 10.1021/es60078a003.
Phillips, G. J., Pouvesle, N., Thieser, J., Schuster, G., Axinte, R., Fischer, H., Williams, J., Lelieveld, J., & Crowley, J. N. (2013). Peroxyacetyl nitrate (PAN) and peroxyacetic acid (PAA) measurements by iodide chemical ionization mass spectrometry: First analysis of results in the boreal forest and implications for the measurement of PAN fluxes. Atmospheric Chemistry Physics, 13, 1129–1139. DOI: 10.5194/acp-13-1129-2013.
Rubio, M. A., Oyola, P., Gramsch, E., Lissi, E., Pizarro, J., & Villena, G. (2004). Ozone and peroxyacetylnitrate in downtown Santiago, Chile. Atmospheric Environment, 38, 4931–4939. DOI: 10.1016/j.atmosenv.2004.05.051.
Stephens, E. R., Darley, E. F., Taylor, O. C., & Scott, W. E. (1961). Photochemical reaction products in air pollution. International Journal of Air and Water Pollution, 4, 79–100.
Tanimoto, H., Hirokawa, J., Kajii, Y., & Akimoto, H. (1999). A new measurement technique of peroxyacetyl nitrate at parts per trilion by volume levels: gas chromatography/negative ion chemical ionization mass spectrometry. Journal of Geophysical Research: Atmospheres, 104, 21343–21354. DOI: 10.1029/1999jd900345.
Tsalkani, N., Perros, P., Dutot, A. L., & Toupance, G. (1991). One-year measurements of PAN in the Paris basin: Efects of meteorological parameters. Atmospheric Environment Part A, General Topics, 25, 1941–1949. DOI: 10.1016/0960-1686(91)90275-c.
von Wandruszka, R. (1992). Luminescence of micellar solutions. Critical Reviews in Analytical Chemistry, 23, 187–215. DOI: 10.1080/10408349208050854.
Zhang, J. M., Wangl T., Dingl A. J., Zhou, X. H., Xue, L. K., Poon, C. N., Wu, W. S., Gao, J., Zuo, H. C., Chen, J. M., Zhang, X. C., & Fan, S. J. (2009). Continuous measurement of peroxyacetyl nitrate (PAN) in suburban and remote areas of western China. Atmospheric Environment, 43, 228–237. DOI: 10.1016/j.atmosenv.2008.09.070.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikuška, P., Bružeňák, L. & Večeřa, Z. Detection of peroxyacetyl nitrate in air using chemiluminescence aerosol detector. Chem. Pap. 68, 1482–1490 (2014). https://doi.org/10.2478/s11696-014-0607-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0607-x