Abstract
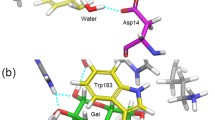
Biguanides are a class of drugs derived from biguanide and they are the most widely used drugs for diabetes mellitus or pre-diabetes treatment. An investigation of their interaction and a transport protein such as β-lactoglobulin (BLG) at atomic level could be a valuable factor in controlling their transport to biological sites. Molecular-docking and molecular dynamics simulation methods were used to study the interaction of metformin, phenformin and buformin as biguanides and BLG as transport protein. The molecular-docking results revealed that these biguanides bind to BLG and that the BLG affinity for binding the biguanides decreases in the following order: phenformin — buformin — metformin. The docking results also show the hydrophobic interactions to have a significant role in the BLG-biguanides complex stability. Analysis of molecular dynamic simulation trajectories shows that the root mean square deviation of various systems attained equilibrium and fluctuated around the mean value at various times. The time evolution of the radius of gyration and the total solvent-accessible surface of the protein showed that BLG and BLG-biguanide complexes became stable at approximately 2500 ps and that there was not any conformational change in the BLG-biguanide complexes. In addition, the profiles of atomic fluctuations show the rigidity of the ligand-binding site during the simulation.
Similar content being viewed by others
References
Alexandrov, V. A., Anisimov, V. N., Belous, N. M., Vasilyeva, I. A., & Mazon, V. B. (1980). The inhibition of the transplacental blastomogenic effect of nitrosomethylurea by postnatal administration of buformin to rats. Carcinogenesis, 1, 975–978. DOI: 10.1093/carcin/1.12.975.
Anisimov, V. N., Ostroumova, M. N., & Dilman, V. M. (1980). Inhibition of the carcinogenic effect of 7,12-dimethylbenz(a) anthracene in female rats by buformin, phenytoin, pineal polypeptide extract and L-dopa. Bulletin of Experimental Biology and Medicine, 89, 819–822. DOI: 10.1007/bf00836263.
Anisimov, V. N. (2003). Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Experimental Gerontology, 38, 1041–1049. DOI: 10.1016/s0531-5565(03)00169-4.
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A., & Haak, J. R. (1984). Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics, 81, 3684. DOI: 10.1063/1.448118.
Berendsen, H. J. C., van der Spoel, D., & van Drunen, R. (1995). GROMACS: A message-passing parallel molecular dynamics implementation. Computer Physics Communication, 91, 43–56. DOI: 10.1016/0010-4655(95)00042-e.
Brownlow, S., Cabral, J. H. M., Cooper, R., Flower, D. R., Yewdall, S. J., Polikarpov, I., North, A. C. T., & Sawyer, L. (1997). Bovine β-lactoglobulin at 1.8 Å resolution — still an enigmatic lipocalin. Structure, 5, 481–495. DOI: 10.1016/s0969-2126(97)00205-0.
Caraci, F., Chisari, M., Frasca, G., Chiechio, S., Salomone, S., Pinto, A., Sortino, M. A., & Bianchi, A. (2003). Effects of phenformin on the proliferation of human tumor cell lines. Life Sciences, 74, 643–650. DOI: 10.1016/j.lfs.2003.07.015.
Darden, T., York, D., & Pedersen, L. (1993). Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. Journal of Chemical Physics, 98, 10089. DOI: 10.1063/1.464397.
Dong, A., Matsuura, J., Allison, S. D., Chrisman, E., Manning, M. C., & Carpenter, J. F. (1996). Infrared and circular dichroism spectroscopic characterization of structural differences between β-lactoglobulin A and B. Biochemistry, 35, 1450–1457. DOI: 10.1021/bi9518104.
Essmann, U., Perera, L., Berkowitz, M. L., Darden, T., Lee, H., & Pedersen, L. G. (1995). A smooth particle mesh Ewald method. Journal of Chemical Physics, 103, 8577. DOI: 10.1063/1.470117.
Flower, D. R., North, A. C. T., & Sansom, C. E. (2000). The lipocalin protein family: structural and sequence overview. Biochimica et Biophysica Acta (BBA) — Protein Structure and Molecular Enzymology, 1482, 9–24. DOI: 10.1016/s0167-4838(00)00148-5.
Jiralerspong, S., Palla, S. L., Giordano, S. H., Meric-Bernstam, F., Liedtke, C., Barnett, C. M., Hsu, L., Hung, M. C., Hortobagyi, G. N., & Gonzalez-Angulo, A. M. (2009). Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. Journal of Clinical Oncology, 27, 3297–3302. DOI: 10.1200/jco.2009.19.6410.
Kontopidis, G., Holt, C., & Sawyer, L. (2002). The ligandbinding site of bovine β-lactoglobulin: Evidence for a function? Journal of Molecular Biology, 318, 1043–1055. DOI: 10.1016/s0022-2836(02)00017-7.
Kontopidis, G., Holt, C., & Sawyer, L. (2004). β-Lactoglobulin: Binding properties, structure, and function. Journal of Dairy Science, 87, 785–796. DOI: 10.3168/jds.s0022-0302(04)73222-1.
Lindah, E., Hess, B., & van der Spoel, D. (2001). GROMACS 3.0: a package for molecular simulation and trajectory analysis. Journal of Molecular Modelling, 7, 306–317. DOI: 10.1007/s008940100045.
Mehraban, M. H., Yousefi, R., Taheri-Kafrani, A., Panahi, F., & Khalafi-Nezhad, A. (2013). Binding study of novel antidiabetic pyrimidine fused heterocycles to β-lactoglobulin as a carrier protein. Colloids and Surfaces B: Biointerfaces, 112, 374–379. DOI: 10.1016/j.colsurfb.2013.08.013.
Nikolic, K., Filipic, S., & Agbaba, D. (2008). QSAR study of imidazoline antihypertensive drugs. Bioorganic & Medicinal Chemistry, 16, 7134–7140. DOI: 10.1016/j.bmc.2008.06.051.
Pullman, B. (1981). Intermolecular forces. Dordrecht, The Netherlands: Reidel.
Renard, D. (1994). Etude de l’agrégation et de la gélification des protéines globulaires: application `a la bétalactoglobuline. Ph.D. thesis, University of Nantes, Nantes, France. (in French)
Roufik, S., Gauthier, S. F., Leng, X. J., & Turgeon, S. L. (2006). Thermodynamics of binding interactions between bovine β-lactoglobulin A and the antihypertensive peptide β-Lg f142-148. Biomacromolecules, 7, 419–426. DOI: 10.1021/bm050229c.
Saito, S., Furuno, A., Sakurai, J., Sakamoto, A., Park, H. R., Shin-ya, K., Tsuruo, T., & Tomida, A. (2009). Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Research, 69, 4225–4234. DOI: 10.1158/0008-5472.can-08-2689.
Sawyer, L., & Kontopidis, G. (2000). The core lipocalin, bovine β-lactoglobulin. Biochimica et Biophysica Acta (BBA) — Protein Structure and Molecular Enzymology, 1482, 136–148. DOI: 10.1016/s0167-4838(00)00160-6.
Schlehuber, S., & Skerra, A. (2005). Lipocalins in drug discovery: From natural ligand-binding proteins to ‘anticalins’. Drug Discovery Today, 10, 23–33. DOI: 10.1016/s1359-6446(04)03294-5.
Schleyer, P. V. R., Allinger, N. L., Clark, T., Gasteiger, J., Kollman, P. A., Schaefer, H. F., III, & Schreiner, P. R. (1998). The encyclopedia of computational chemistry. Chichester, UK: Wiley.
Schmidt, M. W., Baldridge, K. K., Boatz, J. A., Elbert, S. T., Gordon, M. S., Jensen, J. H., Koseki, S., Matsunaga, N., Nguyen, K. A., Su, S. J., Windus, T. L., Dupuis, M., & Montgomery, J. A., Jr. (1993). General atomic and molecular electronic structure system. Journal of Computational Chemistry, 14, 1347–1363. DOI: 10.1002/jcc.540141112.
Schüttelkopf, A. W., & van Aalten, D. M. F. (2004). PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallographica D, 60, 1355–1363. DOI: 10.1107/s0907444904011679.
Thompson, M. A. (2004). Molecular docking using ArgusLab: an efficient shape-based search algorithm and the AScore scoring function. In Proceedings of the Fall ACS Meeting, August 22–26, 2004. Philadelphia, PA, USA: American Chemical Society.
van Gunsteren, W. F., Billeter, S. R., Eising, A. A., Hünenberger, P. H., Krüger, P. K. H. C., Mark, A. E., Scott, W. R. P., & Tironi, I. G. (1996). Biomolecular simulation: The GROMOS96 manual and user guide. Zürich, Switzerland: Vdf Hochschulverlag AG.
Wu, S. Y., Pérez, M. D., Puyol, P., & Sawyer, L. (1999). β-Lactoglobulin binds palmitate within its central cavity. Journal of Biological Chemistry, 274, 170–174. DOI: 10.1074/jbc.274.1.170.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahihi, M., Ghayeb, Y. Binding of biguanides to β-lactoglobulin: molecular-docking and molecular dynamics simulation studies. Chem. Pap. 68, 1601–1607 (2014). https://doi.org/10.2478/s11696-014-0598-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0598-7