Abstract
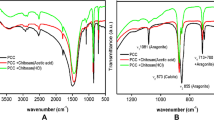
To improve the performance of calcium sulphate whiskers (CSWs) in the papermaking industry, a series of chitosan-coated (CS) calcium sulphate whiskers (CS-CSWs) was prepared, with the aim of effectively reducing their solubility in aqueous solutions. The CS-CSWs were prepared by immersing CSWs in a chitosan acid solution and then cross-linking with alkaline gel via hydrogen-bonding interaction. The CS-CSWs were characterised in terms of water solubility, zeta potential, contact angle, scanning electron microscopy, thermo gravimetric analysis and X-ray photoelectron spectroscopy (XPS). The results indicated that chitosan had a good effect on the surface modification of CSWs. Under optimal conditions, the water solubility of CS-CSWs decreased by 50 %, and the contact angle increased by 63 %. The XPS measurement indicated that the relative thickness of the chitosan coating was 9.8 nm.
Similar content being viewed by others
References
Ashori, A., Harun, J., Zin, W. M., & Yusoff, M. N. M. (2007). Enhancing dry-strength properties of kenaf (Hibiscus cannabinus) paper through chitosan. Polymer-Plastics Technology and Engineering, 45, 125–129. DOI: 10.1080/03602550500373709.
Bahmani, S. A., East, G. C., & Holme, I. (2000). The application of chitosan in pigment printing. Coloration Technology, 116, 94–99. DOI: 10.1111/j.1478-4408.2000.tb00027.x.
Bao, S. H., Fujio, K., & Nomura, T. (2005). Effect of ionic surfactants on the oscillation frequency of one-electrodeseparated piezoelectric quartz crystals modified with chitosan and its derivative. Colloid and Polymer Science, 283, 619–626. DOI: 10.1007/s00396-004-1192-2.
Calvo, P., Remuñán-López, C., Vila-Jato, J. L., & Alonso, M. J. (1997). Development of positively charged colloidal drug carriers: Chitosan-coated polyester nanocapsules and submicron-emulsions. Colloid and Polymer Science, 275, 46–53. DOI: 10.1007/s003960050050.
Faibish, R. S., Yoshida, W., & Cohen, Y. (2002). Contact angle study on polymer-grafted silicon wafers. Journal of Colloid and Interface Science, 256, 341–350. DOI: 10.1006/jcis.2002.8612.
Gan, Q., Wang, T., Cochrane, C., & McCarron, P. (2005). Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids and Surfaces B: Biointerfaces, 44, 65–73. DOI: 10.1016/j.colsurfb.2005.06.001.
Guan, B. H., Ye, Q. Q., Zhang, J. L., Lou, W. B., & Wu, Z. B. (2010). Interaction between α-calcium sulphate hemihydrate and superplasticizer from the point of adsorption characteristics, hydration and hardening process. Cement and Concrete Research, 40, 253–259. DOI: 10.1016/j.cemconres.2009.08.027.
Krajewska, B. (2004). Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme and Microbial Technology, 35, 126–139. DOI: 10.1016/j.enzmictec.2003.12.013.
Leong, K. W., Mao, H. Q., Truong-Le, V. L., Roy, K., Walsh, S. M., & August, J. T. (1998). DNA-polycation nanospheres as non-viral gene delivery vehicles. Journal of Controlled Release, 53, 183–193. DOI: 10.1016/s0168-3659(97)00252-6.
Lim, S. H., & Hudson, S. M. (2003). Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. Journal of Macromolecular Science, Part C: Polymer Reviews, 43, 223–269. DOI: 10.1081/mc-120020161.
Majiti, N. V., & Ravi, K. (2000). A review of chitin and chitosan applications. Reactive and Functional Polymers, 46, 1–27. DOI: 10.1016/s1381-5148(00)00038-9.
Nordtveit, R. J., Vårum, K. M., & Smidsrød, O. (1996). Degradation of partially N-acetylated chitosans with hen egg white and human lysozyme. Carbohydrate Polymers, 29, 163–167. DOI: 10.1016/0144-8617(96)00003-3.
Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31, 603–632. DOI: 10.1016/j.progpolymsci.2006.06.001.
Shanmugasundaram, N., Ravichandran, P., Neelakanta Reddy, P., Ramamurty, N., Pal, S., & Panduranga Rao, K. (2001). Collagen-chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials, 22, 1943–1951. DOI: 10.1016/S0142-9612(00)00220-9.
Sakai, Y., Hayano, K., Yoshioka, H., & Yoshioka, H. (2001). A novel method of dissolving chitosan in water for industrial application. Polymer Journal, 33, 640–642. DOI: 10.1295/polymj.33.640.
Sakai, Y., Hayano, K., Yoshioka, H., Fujieda, T., Saito, K., & Yoshioka, H. (2002). Chitosan-coating of cellulosic materials using an aqueous chitosan-CO2 solution. Polymer Journal, 34, 144–148. DOI: 10.1295/polymj.34.144.
Sloviková, A., Vojtová, L., & Jančař, J. (2008). Preparation and modification of collagen-based porous scaffold for tissue engineeringt. Chemical Papers, 62, 417–422. DOI: 10.2478/s11696-008-0045-8.
Wang, X. L., Zhu, Y. M., Han, Y. X., Yuan, Z. T., & Yin, W. Z. (2009). Toughening of polypropylene with calcium sulfate whiskers treated by coupling agents. Advanced Materials Research, 58, 225–229. DOI: 10.4028/www.scientific.net/amr.58.225.
Wang, Y. W., Kim, Y. Y., Christenson, H. K., & Meldrum, F. C. (2012). A new precipitation pathway for calcium sulfate dihydrate (gypsum) via amorphous and hemihydrate intermediates. Chemical Communications, 48, 504–506. DOI: 10.1039/c1cc14210k.
Xu, A. Y., Li, H. P., Luo, K. B., & Xiang, L. (2011). Formation of calcium sulfate whiskers from CaCO3-bearing desulfurization gypsum. Reserch on Chemical Intermediates, 37, 449–455. DOI: 10.1007/s11164-011-0283-1.
Zhao, K., Asami, K., & Lei, J. (2002). Dielectric analysis of chitosan microsphere suspensions: study on its ion adsorption. Colloid and Polymer Science, 280, 1038–1044. DOI: 10.1007/s00396-002-0730-z.
Zhao, Z. P., Wang, Z., & Wang, S. C. (2003). Formation, charged characteristic and BSA adsorption behavior of carboxymethyl chitosan/PES composite MF membrane. Journal of Membrane Science, 217, 151–158. DOI: 10.1016/s0376-7388(03)00105-4.
Zhang, M. G., Smith, A., & Gorski, W. (2004). Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Analytical Chemistry, 76, 5045–5050. DOI: 10.1021/ac049519u.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Q., Cheng, Y., Cao, XY. et al. Preparation and physical properties of chitosan-coated calcium sulphate whiskers. Chem. Pap. 68, 1400–1407 (2014). https://doi.org/10.2478/s11696-014-0579-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0579-x